Article Text
Abstract
Background To compare the efficacy of two different concepts of cisternal therapy—PREVENTIVE fibrinolysis plus on-demand spasmolysis versus RESCUE spasmolysis—for the prevention of cerebral vasospasm (CVS) and delayed cerebral infarction (DCI) in patients with aneurysmal subarachnoid haemorrhage (aSAH).
Methods Retrospective analysis of 84 aSAH patients selected for cisternal therapy for DCI prevention. 66 high-risk patients received PREVENTIVE cisternal therapy to enhance blood clearance. Either stereotactic catheter ventriculocisternostomy (STX-VCS) or intraoperative placement of a cisterno-ventriculostomy catheter (CVC), followed by fibrinolytic cisternal lavage using urokinase was performed. In case of vasospasm, nimodipine was applied intrathecally. 22 low-risk patients who developed CVS against expectations were selected for STX-VCS as RESCUE intervention for cisternal spasmolysis with nimodipine. Rates of DCI and mean flow velocities of daily transcranial Doppler (TCD) ultrasonographies were evaluated.
Results Despite a higher prespecified DCI risk, patients selected for PREVENTIVE intervention primarily aiming at blood clearance had a lower DCI rate compared with patients selected for intrathecal spasmolysis as a RESCUE therapy (11.3% vs 18.2%). After intrathecal treatment onset, CVS (TCD>160 cm/s) occurred in 45% of patients with PREVENTIVE and 77% of patients with RESCUE therapy (p=0.013). A stronger response of CVS to intrathecal nimodipine was observed in patients with PREVENTIVE intervention as the mean CVS duration after start of intrathecal nimodipine was 3.2 days compared with 5.8 days in patients with RESCUE therapy (p=0.026).
Conclusions PREVENTIVE cisternal therapy directed at blood clearance is more effective for the prevention of CVS and delayed infarction compared with cisternal RESCUE spasmolysis.
Trial registration number DRKS00016532.
- subarachnoid
- stroke
- aneurysm
- cerebral infarction
Data availability statement
Data are available on reasonable request. Data from this study are available on reasonable request and in accordance with European data protection rules.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Delayed cerebral infarction (DCI) is an important contributor to the high morbidity and fatality of aneurysmal subarachnoid haemorrhage (aSAH).1 Neither preventive nor therapeutic strategies to reduce the burden of DCI reaching level-I evidence are available.2
Intrathecal therapies directed at clearance of intracranial blood or dilation of vasospastic vessels represent promising DCI therapies.3
We introduced intrathecal therapies into aSAH management in 2015. In principle, two different concepts were applied. Patients at high risk for DCI (ie, poor clinical grade and high amount of blood) were selected for PREVENTIVE intrathecal therapy: cisternal catheters were placed during (cisterno-ventriculostomy through the fenestrated lamina terminalis, cisterno-ventriculostomy catheter, CVC)4 or soon after (stereotactic catheter-ventriculocisternostomy, STX-VCS)5 aneurysm securing. Fibrinolytic lavage was applied using urokinase to enhance blood clearance. In case of vasospasm, patients received intrathecal nimodipine for spasmolysis. In contrast, patients at low DCI risk who developed cerebral vasospasm (CVS) against expectations were selected for RESCUE intrathecal therapy: STX-VCS was performed for cisternal application of nimodipine.6
Here, we compare the efficacy of these two different concepts of intrathecal therapy aiming at DCI prevention.
Patients and methods
Data from this study are available on reasonable request and in accordance with European data protection rules. The study took place in the neurosurgical department of a tertiary referral centre. It was performed according to the Declaration of Helsinki and is reported in accordance with institutional guidelines. Patients admitted before January 2019 were retrospectively included and informed consent was waived by our independent ethics committee. Patients admitted as of January 2019 were enrolled in our prospective aSAH registry (registered at the German Clinical Trials Register: DRKS00016532) and provided informed consent.
The study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting of observational studies.7
A total of 281 aSAH patients were admitted to our department between October 2015 and October 2020. Eighty-four (29.9%) of these patients were selected for intrathecal therapy on the basis of individual treatment decisions. Patients who were enrolled in a randomised controlled trial for early STX-VCS8 which started recruitment in July 2019 were excluded.
Baseline information including patient demographics (age, sex), aneurysm size and location (categorised in: anterior cerebral artery, middle cerebral artery, internal carotid artery (ICA) - including posterior communicating artery and posterior circulation artery, World Federation of Neurosurgical Societies (WFNS) and modified Fisher scores were collected. The amount of cisternal and ventricular blood on the initial cranial CT was measured semiquantitatively using the Hijdra scale.9
Presence or absence of DCI as visualised by cranial imaging was recorded applying the Vergouwen criteria.10 Independent assessment of DCI was performed by a rating board. The prespecified DCI risk of all patients was estimated by application of the de Rooij score.11
Methods of cisternal lavage
Two surgical methods for obtaining catheter access to the basal cisterns and apply cisternal lavage were used as previously reported.4 5
In patients with (1) aneurysm coiling or (2) RESCUE intervention a stereotactic catheter ventriculocisternostomy (STX-VCS) was performed. Stereotactic procedures were performed using a Leksell G-Frame (Elekta, Stockholm, Sweden). A right or left frontal twist drill burr hole (3.5 mm) was performed under stereotactic guidance. Standard EVD catheters (typically 2.8 mm diameter) were stereotactically implanted via the lateral ventricle, the foramen of Monroi, the third ventricle, perforating the floor and entering the prepontine cistern, creating a third ventriculocisternostomy.
A CVC for PREVENTIVE intrathecal therapy was placed via the fenestrated lamina terminalis in patients who were (A) considered at high risk for DCI and (B) underwent clipping of aneurysms that required access to the chiasmatic region nevertheless (typically: clipping of aneurysms of the anterior communicating artery). A standard EVD catheter running through the sylvian fissure was advanced into the third ventricle. To allow continuous cisternal lavage, both catheter approaches were combined with an external ventricular drainage. A free running electrolyte solution at a rate of 50 mL/hour was used as carrier solution for cisternal lavage.
Patients selected for a PREVENTIVE intervention received Urokinase (medac GmbH, Wedel, Germany) at a concentration of 100 IU/mL until macroscopic clearance of drainage fluid was observed, typically for the first 7–14 days. Nimodipine (Bayer Vital, Leverkusen, Germany) at a concentration of 0.01 mg/mL was used in case of clinical signs of delayed neurological deficit or sonographic vasospasm (defined as maximum mean flow velocity (MFV) of either intracranial artery exceeding 160 cm/s). Drug-free irrigation was continued according to the opinion of the treating intensive care physicians, typically until days 14–21 after aSAH.
Patients selected for RESCUE intervention received nimodipine at a concentration of 0.01 mg/mL.
Statistical analyses
Baseline characteristics are presented as means±SD or SE, medians with IQR or frequencies (%), as appropriate. Differences in baseline characteristics between groups were assessed using the Mann-Whitney U test, Fisher’s exact test or Pearson χ2 test, as appropriate. The endpoints of the primary analyses were differences in DCI and the burden of CVS/nimodipine response. Differences are reported as relative risks with 95% CIs. The significance level was set at alpha ≤0.05 for all statistical analyses. Statistical analyses were generated using GraphPad Prism V.7 (GraphPad Software, San Diego, USA).
Results
Baseline characteristics
Eighty-four (29.9%) of 281 patients with aSAH admitted between October 2015 and October 2020 were selected for intrathecal therapies on the basis of individual treatment decisions. Sixty-two (74%) were selected for PREVENTIVE intervention with the primary goal of intracranial blood clearance using fibrinolytic (urokinase) lavage. Two methods to deliver cisternal lavage were applied in patients selected for PREVENTIVE intervention: 51 patients received a stereotactic catheter-ventriculocisternostomy (STX-VCS). In 11 patients, a CVC running through the sylvian fissure into the third ventricle via the fenestrated lamina terminalis was placed during microsurgical clipping of the ruptured aneurysm. Twenty-two low-risk patients who developed CVS against expectations were selected for intrathecal RESCUE therapy and STX-VCS was performed to apply nimodipine intracisternally.
Clinical and demographic characteristics of PREVENTIVE and RESCUE patient cohorts are shown in table 1. Patients selected for PREVENTIVE intervention were older (61 vs 52 years), had worse admission WFNS grades, higher intracranial blood amounts (Hijdra sum scale: 26.3 vs 16.5), and differed in aneurysm location from patients with RESCUE intervention. Accordingly, the prespecified DCI-risk (de-Rooij score) for patients with PREVENTIVE intervention was significantly higher compared with patients with RESCUE intervention (47.5% vs 37.7%).
Clinical characteristics of aSAH patients with preventive and rescue intrathecal therapy
The mean time between aSAH onset and the intervention for cisternal lavage therapy was 53 hours (2.2 days) in patients with PREVENTIVE intervention and 161 hours (6.7 days) in patients with RESCUE intervention.
Four (18%) patients in the RESCUE cohort underwent endovascular therapy for CVS. In all cases, these interventions were performed before intrathecal therapy was commenced. Two (3%) patients in the PREVENTIVE cohort underwent endovascular interventions for CVS. These interventions were performed after initiation of intrathecal therapy.
Intrathecal drug use
Table 2 summarises the use of intrathecal drugs in both groups. According to the respective concept of intrathecal therapy, 98% of patients selected for PREVENTIVE intervention received intrathecal urokinase and 100% of patients selected for RESCUE therapy received nimodipine. One patient with PREVENTIVE intervention was immediately treated with nimodipine. Vice versa, 58% of patients with PREVENTIVE intervention developed CVS and—on demand—received nimodipine. Fibrinolytic therapy was performed in only 14% of patients with RESCUE intervention and was applied after cessation of CVS to enhance blood clearance. In the same sense, opposing use durations of Urokinase and nimodipine were used in both groups.
Intrathecal drug use
Cerebral vasospasm
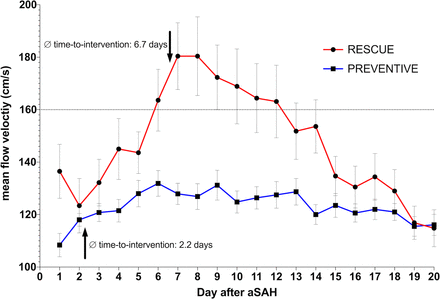
The mean MFV on the first 20 days after aSAH in patients with PREVENTIVE and RESCUE intervention differed significantly between day 4 and 14 since (1) patients with PREVENTIVE intervention did not show the aSAH characteristic MFV peak around days 7–10 and (2) MFV was not immediately normalised after the intervention in patients with RESCUE therapy (figure 1).
Mean maximum mean flow velocity (MFV) in patients with PREVENTIVE and RESCUE intervention. Mean (±SE) daily maximum MFV in transcranial Doppler ultrasonography of patients with PREVENTIVE (blue line) and RESCUE (red line) intervention. The mean MFV was significantly lower in patients with PREVENTIVE intervention on days 4 through 14. Patients with PREVENTIVE intervention lacked the characteristic peak increase of MFV around day 5–10. aSAH, aneurysmal subarachnoid haemorrhage.
Burden of vasospasm
Figure 2 shows the respective percentage of patients with sonographic vasospasm in both groups on the first 20 days after aSAH.
Percent of patients with vasospasm: PREVENTIVE versus RESCUE therapy. Percentage of patients with PREVENTIVE and RESCUE intervention with MFV exceeding 160 cm/s (critical vasospasm) on the first 20 days after aSAH. aSAH, aneurysmal subarachnoid haemorrhage; MFV, mean flow velocities; TCD, transcranial Doppler.
Table 3 summarises the rates and durations of sonographic vasospasm in both groups before and after the intervention for intrathecal therapy. Overall, 29 of 62 patients (47%) selected for PREVENTIVE intervention ever had sonographic vasospasm (MFV increase to 160 cm/s or higher). Twenty of 22 patients (91%) selected for RESCUE therapy ever had sonographic vasospasm. Two patients had clinical vasospasm/delayed neurological deterioration. The duration of vasospasm was considerably longer both before and after intervention in the RESCUE therapy group.
Burden of vasospasm (days with TCD >160 cm/s preintervention and postintervention)
Response of CVS to intrathecal nimodipine
To compare the response of CVS to intrathecal nimodipine in patients with PREVENTIVE versus RESCUE therapy, we normalised the transcranial Doppler (TCD) ultrasonographies (TCD) values to the time point of first ever intrathecal nimodipine administration. For this analysis, we excluded patients who did not receive intrathecal nimodipine (ie, 26 patients from the PREVENTIVE group) (figure 3). In patients with PREVENTIVE intervention, nimodipine start led to a rapid reduction of CVS: the mean TCD values decreased to subcritical levels on the following day. Remarkably, the response of CVS to a RESCUE application of nimodipine was considerably weaker: the mean TCD values remained above 160 cm/s for 5 days. Accordingly, the burden of vasospasm was substantially higher in patients with RESCUE therapy.
Response of cerebral vasospasm to intrathecal nimodipine. Daily mean MFV of patients with PREVENTIVE and RESCUE intervention normalised to the day of first-ever intrathecal nimodipine application. MFV, mean flow velocities.
Delayed cerebral infarction
The observed DCI rates in patients with PREVENTIVE and RESCUE intervention were 11.3% (7 of 62 patients) and 18.2% (4 of 22 patients), respectively. For both groups, this was below the prespecified DCI-risk according to the de Rooij score (figure 4).
Prespecified versus observed DCI rates in patients with PREVENTIVE and RESCUE intervention. The de Rooij score (expected DCI rate) for both groups was calculated and compared with the observed DCI rate. DCI, delayed cerebral infarction.
Discussion
Our experience with both a PREVENTIVE intrathecal intervention primarily directed at blood clearance and a RESCUE intervention directed at intrathecal spasmolysis shows that addressing the root cause of CVS—cisternal and ventricular blood—by PREVENTIVE cisternal fibrinolysis is more effective for the prevention of CVS and DCI than intrathecal RESCUE spasmolysis.
In keeping with the pertinent literature, both treatment concepts were associated with a significant reduction of the DCI rate. A ca. 50% reduction was observed for patients selected for RESCUE therapy. Patients selected for PREVENTIVE intervention had an extremely high prespecified risk for DCI. Yet, only half of these patients ever developed CVS and the observed DCI rate was less than one quarter of the expected DCI rate.
We observed large differences in the response of CVS to intrathecal nimodipine: In patients selected for PREVENTIVE intervention intrathecal nimodipine application resulted in a rapid resolution of CVS. In contrast, CVS responded considerably slower if cisternal nimodipine was applied as a RESCUE intervention. We argue that two main mechanisms contribute to this difference in nimodipine response.
First, the time between detection of CVS and the first application of Nimodipine was shorter in patients with PREVENTIVE intervention. Any detection of (clinical or sonographic) CVS promptly triggered the application of nimodipine since a treatment access to the basal cisterns was already in place. In turn, the decision to perform a surgical RESCUE intervention was commonly preceded by several days of increasing CVS but the intervention was withheld until CVS was considered critical. We argue that CVS consolidates all the longer it is present. Hence, cerebral arteries affected by CVS for longer times may respond much less to spasmolytic agents. This hypothesis is supported by histological and functional findings in vasospastic arteries in both human and animal studies: short-lasting vasospasm is associated with only subtle structural changes of arterial walls. Chronic vasospasm is associated with myonecrotic changes in the blood vessel wall leading to narrowing, rather than a sustained myovascular contraction.12–14 In consequence, chronically vasospastic cerebral arteries show a (receptor-indendent) depressed contractile response to all vasoactive substances.15
Second, if no fibrinolysis has been performed, surrounding blood clots may impede intrathecally administered nimodipine to reach its target of action and, thereby, limit spasmolytic effectivity.
In summary, intrathecal therapies represent promising approaches to reduce DCI in patients with aSAH and various approaches have found clinical application.3 Both fibrinolytic and spasmolytic therapies have been used and remain under investigation. The basal cisterns appear to be the most promising target for delivery of such therapies since cisternal fibrinolysis successfully reduced DCI16 but both intraventricular fibrinolysis17 and spasmolysis18 have failed to prevent DCI. Our results underscore the importance of a preventive treatment approach and indicate that early cisternal blood clearance is a powerful method for DCI prevention. Reacting to established CVS by cisternal spasmolysis is less effective. Accordingly, a randomised clinical trial to assess safety and efficacy of PREVENTIVE cisternal therapy is recruiting (EudraCT 2017-000868-15).8
Study limitations
Our study is subject to the general constraints of retrospective analyses. We have tried to exclude potential bias by independent assessment of important endpoints (DCI). The baseline characteristics of the two groups analysed in this paper are different, making a direct comparison difficult. A validated method for estimating the DCI risk (de Rooij score) was therefore used to compare the capacity of the two treatment strategies for DCI prevention.
Conclusions
Preventive cisternal therapy directed at blood clearance is more effective for the prevention of CVS and delayed infarction compared with cisternal rescue spasmolysis.
Data availability statement
Data are available on reasonable request. Data from this study are available on reasonable request and in accordance with European data protection rules.
Ethics statements
Patient consent for publication
Ethics approval
The study was approved by the independent Ethics Committee, Medical Centre-University of Freiburg, Germany; reference numbers: 575/16, 184/18.
Acknowledgments
We thank Dr Beate Hippchen for excellent data base management.
References
Footnotes
Contributors RR: study conceptualisation, data collection and interpretation, statistical analyses, visualisation, drafting of the manuscript. RR is responsible for the overall content as the guarantor. RR accepts full responsibility for the work and the conduct of the study, had access to the data and controlled the decision to publish. CS: data curation, study supervision, interpretation of data, reviewed the manuscript for important intellectual content. JG: data curation, reviewed the manuscript for important intellectual content. IC: data collection and analysis, reviewed the manuscript for important intellectual content. VAC: study supervision, reviewed the manuscript for important intellectual content. JB: study conceptualisation, data curation, project administration, reviewed the manuscript for important intellectual content. PCR: study conceptualisation, collection and interpretation of data, drafting of the manuscript.
Funding RR is funded by the Berta-Ottenstein-Programme for Advanced Clinician Scientists, Faculty of Medicine, University of Freiburg.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.