Article Text
Abstract
Introduction The safety outcomes of endovascular therapy for intracranial artery stenosis in a real-world stetting are largely unknown. The Clinical Registration Trial of Intracranial Stenting for Patients with Symptomatic Intracranial Artery Stenosis (CRTICAS) was a prospective, multicentre, real-world registry designed to assess these outcomes and the impact of centre experience.
Methods 1140 severe, symptomatic intracranial arterial stenosis (ICAS) patients treated with endovascular therapy were included from 26 centres, further divided into three groups according to the annual centre volume of intracranial angioplasty and stent placement procedures over 2 years: (1) high volume for ≥25 cases/year; (2) moderate volume for 10–25 cases/year and (3) low volume for <10 cases/year.
Results The rate of 30-day stroke, transient ischaemic attack or death was 9.7% (111), with 5.4%, 21.1% and 9.7% in high-volume, moderate-volume and low-volume centres, respectively (p<0.05). Multivariable logistic regression confirmed high-volume centres had a significantly lower primary endpoint compared with moderate-volume centres (OR=0.187, 95% CI: 0.056 to 0.627; p≤0.0001), while moderate-volume and low-volume centres showed no significant difference (p=0.8456).
Conclusion Compared with the preceding randomised controlled trials, this real-world, prospective, multicentre registry shows a lower complication rate of endovascular treatment for symptomatic ICAS. Non-uniform utilisation in endovascular technology, institutional experience and patient selection in different volumes of centres may have an impact on overall safety of this treatment.
- angioplasty and stenting
- atherosclerosis
- intracranial stenosis
- outcome
- prospective registry
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Stroke is the second leading cause of death worldwide, and intracranial arterial stenosis (ICAS) accounts for 10%–54% of all ischaemic strokes.1 The results of the only two multicentre randomised controlled trials (RCTs), designed to assess the efficacy of endovascular therapy versus medical treatment for ICAS, did not support endovascular therapy.2 3 However, criticisms have been raised regarding their designs and generalisability.4 Two recent studies with tailored patient selection criteria reported complication rates from 4.3% to 5.0%,5 6 considerably lower than the results of the the Vitesse Intracranial Stent Study for Ischemic Stroke Therapy (VISSIT: 24.1%) and the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS: 14.7%) trials.2 3 Therefore, it has been argued in the wake of these new studies that the real-world safety outcomes of endovascular therapy may still be unknown. Site experience, reflected as centre volume, was suggested to be highly related to safety.7
Thus, we designed the prospective multicentre Clinical Registration Trial of Intracranial Stenting for Patients with Symptomatic Intracranial Artery Stenosis with aims to evaluate the safety of endovascular therapy in patients with severe symptomatic ICAS in a real-world situation and clarify the impact of centre volume on the safety outcomes of endovascular therapy for ICAS.
Methods
Study design
This was a prospective, real-world registry with 26 participating centres, which were further divided into three groups according to the annual centre volume of intracranial angioplasty and stenting procedures over 2 years: (1) high volume for ≥25 cases/year; (2) moderate volume for 10–25 cases/year and (3) low volume for <10 cases/year.
Patient enrolment
Inclusion criteria were: (1) 30–80 years of age; (2) 70%–99% stenosis of a major intracranial artery (internal carotid artery (ICA), middle cerebral artery (MCA) (M1 segment), vertebral artery (VA), basilar artery (BA)) measured by digital subtraction angiography using the standard warfarin-aspirin symptomatic intracranial disease method8; (3) target vessel measuring 2–4.50 mm in diameter with the lesion≤14 mm in length and (4) symptoms included transient ischaemic attack (TIA) or minor ischaemic stroke within the past 90 days but not including the most recent 21 days as the risk of haemorrhagic transformation from a procedure was felt to be too high. Exclusion criteria were: (1) acute infarct within 3 weeks (21 days) in view of haemorrhagic transformation risk; (2) intracranial haemorrhage in the territory of the stenotic artery, brain infarct of sufficient size (>5 cm on CT/MRI) within 15 days or previous spontaneous intracranial haemorrhage within 30 days and (3) baseline modified Rankin Scale>3; 4) stenosis caused by non-atherosclerotic lesions or concurrent intracranial tumours, aneurysms or vascular malformations.
Procedures and medical management
Therapy was left to the discretion of the neurointerventionalist to select one of: balloon-mounted stent (BMS: Apollo), self-expanding stent (SES: Gateway balloon plus the Wingspan stent system, Solitaire AB stent system or Enterprise stent system) or primary transluminal angioplasty (PTA: Gateway) without stenting. All patients received aspirin (100 mg/day) and clopidogrel (75 mg/day) for at least 5 days before the procedure. Dual antiplatelet therapy was continued for 3 months thereafter.
Outcomes
The primary outcome was a composite of any stroke (including ischaemic or haemorrhagic stroke), TIA or death within 30 days after endovascular therapy. Secondary outcomes were ischaemic stroke, haemorrhagic stroke or death within 30 days after endovascular therapy.
Statistical analysis
Prespecified intention-to-treat analysis was employed. Continuous variables were presented as the mean±SD and compared with Analysis of Variance (ANOVA) tests. Categorical variables were presented as numbers and frequencies and compared with χ2 tests or Fisher’s exact tests, as appropriate. For the primary outcome, multiple logistic regression analysis was performed. All demographic and clinical characteristics of the patients were included in stepwise regression analysis using SAS V.9.2 software.
Results
Patient characteristics
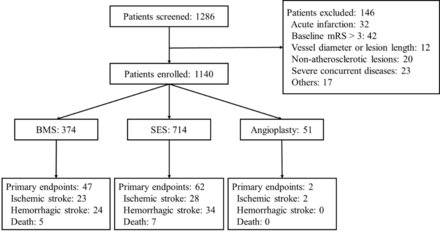
From December 2013 to December 2015, 1286 patients with symptomatic ICAS were consecutively screened in 26 participating centres and 1140 patients were ultimately enrolled. Overall, 774 (67.9%) patients were treated in high-volume centres, 294 (25.8%) patients were treated in moderate-volume centres and 72 (6.3%) patients were treated in low-volume centres (table 1, figure 1). Patients were treated with BMS in 375 (32.9%) of cases, SES in 714 (62.6%) and PTA in 51 (4.5%).
Design and flow of the Clinical Registration Trial of Intracranial Stenting for Patients with Symptomatic Intracranial Artery Stenosis Study. BMS, balloon-mounted stent; mRS, modified Rankin Scale; SES, self-expanding stent.
Baseline characteristics and 30-day safety outcomes
Primary and secondary endpoints
The primary endpoint occurred in 9.7% (111/1140, table 2) of the included patients. Haemorrhagic stroke and ischaemic stroke occurred in 58 (5.1%) and 53 (4.7%) patients, respectively. The 30-day mortality was 1.1% (12/1140). For ischaemic strokes, 8 (0.7%) patients were related to in-stent thrombosis and 41 (3.6%) patients related to perforator occlusion. For haemorrhagic strokes, 28 (2.5%) patients had subarachnoid haemorrhage and 30 (2.6%) patients had symptomatic intracranial/intraparenchymal haemorrhage. The rates of primary endpoint occurrence were significantly different between volume categories (p<0.0001). High-volume centres had the lowest rate at 5.4%, compared with moderate-volume or low-volume centres at 9.7%–21.1%.
Analyses of 30-day safety endpoints by centre volume
Multivariable logistic regression analysis
After controlling for therapy and patient-level factors, high-volume centres were significantly associated with better primary outcomes compared with moderate-volume centres (OR=0.187, 95% CI: 0.056 to 0.627; p≤0.0001), while no statistical difference was detected between moderate-volume and low-volume centres (p=0.8456) (table 3). PTA alone was related to the lowest rate of the primary endpoint compared with stenting method (OR=0.591, 95% CI: 0.073 to 4.790; p≤0.0001). MCA location was significantly related to lower rates of the primary endpoint (OR=0.050, 95% CI: 0.016 to 0.158; p≤0.0001), while ICA and VA locations were related to higher rates of the primary endpoint (ICA: OR=4.579, 95% CI: 1.539 to 13.625; p≤0.0001; VA: OR=1.453, 95% CI: 0.588 to 3.589; p=0.0167). Arterial morphology indicated by Mori classification was also associated with significantly differing rates of the primary endpoint, with Mori A being the lowest (p=0.0167).
Multivariate regression of 30-day safety outcomes
Discussion
To our knowledge, this is the largest registry evaluating the real-world safety of endovascular therapy following the SAMMPRIS trial. This study reported a 9.7% rate of the primary outcome, which is lower than the rate reported in the SAMMPRIS and VISSIT (14.7% and 24.1%, respectively) trials (online supplemental file 1),2 3 while still higher than the rate of 2.6% in the Wingspan Stent System Post Market Surveillance (WEAVE) trial9 and 2.0% in the lead-in phase of the China Angioplasty and Stenting for Symptomatic Intracranial Severe Stenosis trial.10 These RCTs had strict inclusion criteria which arguably limited their generalisability to the real-world state of neuroendovascular therapy for ICAS. Unmistakably, this rate is still higher than the best medical therapy arms of the landmark trials, but these data suggest that endovascular therapy for symptomatic ICAS may yet hold promise for the medication-refractory subpopulation, acknowledging that medical management has also improved since the major trials.
Supplemental material
The WEAVE trial was an FDA mandated postmarket surveillance study of the Wingspan stent for on-label usage.9 Both the WEAVE trial and the present study were based on real-world data to evaluate endovascular therapy for symptomatic ICAS, but yielded different primary outcomes (30-day rate of stroke and death: 2.6% vs 9.7%). First, the WEAVE trial enrolled only on-label patients, while the present study additionally enrolled off-label usage of Wingspan and other types of stents. Second, the WEAVE trial used strict criteria to select experienced interventionalists and high-volume participating sites, while the present study did not set eligibility criteria for centre volume and the present data were not enough for further analysis for operator experience. Third, medical treatment and risk factor control may also be different between two studies. For example, antiplatelet resistance testing was not a routine test in the present study due to its relative expense. Compliance with medications was not evaluated in the present study and may differ from that in the WEAVE trial. Further studies are needed to confirm the results obtained. In real-world practice, endovascular therapy for patients with ICAS is performed in numerous stroke centres with differing volumes. Centre volume was significantly associated with the primary endpoint and patients treated in high-volume centres had a better 30-day prognosis than those treated in middle-volume or low-volume centres as suspected by smaller recent studies.11 12 Importantly, centre experience comprises preprocedural evaluation, management of comorbidities and risk factors and periprocedural care, among other crucial attributes beyond just operator experience.
As shown in table 1, BMS was more frequently used in high-volume centres than SES (83.5% vs 57.6%). This unequal distribution may be related to more experience in dealing with more complex devices (BMS over SES) in high-volume centres, which needs confirmation by future studies. It may be that use of with BMS is more complex than SES, given more complex access, navigation of more rigid material or different device sizing. Further studies are needed to confirm this speculation. In the multivariable regression analysis, both type of stent and centre volume were included to study their association with the primary outcome (table 3). No significant difference in the primary outcome was shown between different types of stents in the multivariable regression analysis (p=0.0612).
Location of stenosis and arterial morphology were significantly associated with endovascular therapy outcome as MCA location and Mori A lesions were protective factors, while ICA location and Mori C lesions were risk factors. The results related to lesion location in the present study had two main features. First, lesions in posterior circulation were more frequent than those in anterior circulation, which is similar to a previous study in China13 but different from studies from western countries.2 Second, BA and MCA locations had a lower complication risk than VA and ICA, respectively, which was different from the previous study.14 We performed an additional analysis, which also showed that MCA location was related to lower risk of primary endpoints compared with ICA (OR 0.011, 95% CI 0.003 to 0.039; p<0.0001). The results were contrary to those from a previous retrospective study, in which periprocedural symptomatic ischaemic strokes occurred significantly more often in patients with posterior versus anterior ICAS treatment (14.5 vs 5.1%, p=0.048). Periprocedural ischaemic strokes were predominantly perforator strokes (73.3%), which may be caused by the ‘snowplow effect’, although the exact reason is unknown.15 We speculate that this may also be related to selection bias in this real-world study. Further studies are needed to confirm this. Additionally, choice of endovascular therapy resulted in different 30-day safety outcomes. Here, 3.9% of patients experienced stroke/TIA or death in the PTA group, which was the lowest compared with the two stents groups (p=0.0450; table 1). PTA alone is a relatively simple and rapid procedure compared with stenting and avoids long-term risk of stents implement, which may account for the risk of complication in PTA group. Lastly, the primary outcome was significantly related to the centre volume, which likely reflects comprehensiveness of care. However, both low volume and PTA were less represented due to relatively small sample size. Operators tended to choose stents over PTA alone, perhaps previous studies have suggested that PTA alone was related to a greater risk of restenosis and acute thrombosis.
Limitations
Our multicentre study involved one country and generalisability may be limited. We attempted to offset this by conducting a real-world investigation, although this may inherently not be as data-complete as an RCT. Relatedly, ICAS accounts for 30%–50% and 8%–9% of ischaemic events in Asians and Caucasians, respectively. This was a single-arm interventional registry without a control medical arm which focused on short-term safety outcomes and thus long-term data are not available.
Conclusion
This prospective multicentre registry demonstrated a lower complication rate in treating patients with symptomatic ICAS with endovascular therapy in a real-world context, compared with the preceding RCTs. Uneven development in endovascular technology, institutional experience and patient selection in different volumes of centres may have an impact on overall safety of this treatment.
Ethics statements
Patient consent for publication
Ethics approval
This study was registered at blinded for peer-review and approved by the research ethics committee of each participating centre.
References
Supplementary material
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @AdamDmytriw
Contributors Contributed equally to this article, data analysis and drafting the manuscript: YW and TW. Study concept and design: LJ, YW and TW. Data acquisition and verification: all authors.
Funding This work was supported by the National Key Research and Development Project (2016YFC1301703) and the Beijing Scientific and Technologic Project (D161100003816002).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.