Article Text
Statistics from Altmetric.com
As several randomized trials have shown the overwhelming benefit of endovascular treatment (EVT) compared with medical management alone for acute ischemic stroke (AIS) due to large vessel occlusion (LVO),1 EVT has become an integral part of LVO treatment.2 For medium vessel occlusions (MeVOs)—that is, M2/3, A2/3, and P2/3 segment occlusions—the situation is less clear.
What we know and don’t know about MeVOs
We know that the natural history of M2 occlusions is poor. Despite the relatively distal occlusion site, only half of patients with M2 occlusions achieve a good functional outcome at 90 days.3 The clinical course of M3 occlusions and occlusions of the A2/3 and P2/3 segments is unknown, partly because vascular imaging was not routinely performed for AIS in the pre-EVT era, and even if it was, detecting MeVOs can be challenging, particularly for physicians with little imaging experience. Technically, imaging tools to detect MeVOs, such as perfusion imaging and multiphase CT angiography,4 have been available for many years, even before devices that render safe and responsible EVT in MeVOs possible. However, it is only recently that innovative postprocessing methods, such as time variant multiphase CT angiography color maps,5 which further facilitate MeVO detection for unexperienced readers, have become available. Since MeVO patients were underrepresented in previous EVT trials (the MulticenterRandomized Clinical trial of Endovascular treatment for Acute ischemic stroke in the Netherlands (MR CLEAN) trialwas the only trial that included patients with M2 occlusions, and they constituted only 8% of the trial’s patient population),6 there is no clear guideline based EVT recommendation for MeVO strokes.2 7 We have limited evidence for the safety and efficacy of EVT in M2 occlusions from the HERMES collaboration, but these data are not sufficient to change treatment guidelines. At present, such a change requires high level evidence from one, or ideally several, randomized controlled trials.
The challenge of defining MeVOs
Even for LVOs, it is not entirely clear how they should be defined.8 Varying criteria are used to distinguish the M1 segment, which is considered a 'large vessel', from the M2 segment, which is not included in the traditional definition of 'LVO'. When defining MeVOs, the situation is even more complex. Conceptually, primary MeVOs have to be distinguished from secondary MeVOs, which can result from iatrogenic thrombus fragmentation during EVT or as a consequence of clot migration, especially after intravenous alteplase administration.9 In the following, we refer to primary MeVOs. Broadly speaking, there are three features that can be used as reference points to define these: (a) vessel anatomy, (b) vessel size, and (c) clinical deficit. While the former two are based on morphology, the latter is purely functional. Merely relying on vessel anatomy or vessel size has its limitations, as there is a high degree of interindividual variability. A definition exclusively based on functional deficits also seems impractical, as patients with LVOs can have relatively mild symptoms if they have good collateral supply, whereas even MeVOs can be severely disabling depending on the eloquence of the affected tissue. Thus a comprehensive definition of MeVOs will most likely include both anatomical and functional aspects.
We suggest a definition of MeVO (table 1) and provide a perspective on EVT in these patients.
Proposed definition of medium vessel occlusion (both A and B have to apply)*
How physicians think about MeVOs
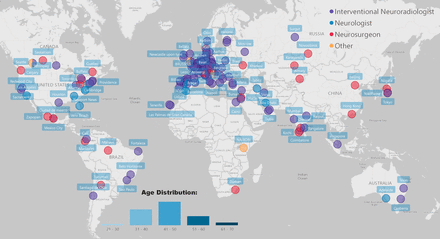
Current level 1A EVT recommendations for AIS are restricted to patients with LVO.2 7 Clinical practice however often precedes guideline recommendations. For instance, most physicians around the world would routinely offer EVT for AIS due to M2 occlusions.10 Less is known about EVT preferences in patients with M3 occlusions, and those with occlusions of the anterior and posterior cerebral arteries. We conducted a multinational survey (ESCAPE ALICE) among neurointerventionalists (n=184, figure 1) to explore how physicians approach such cases. Survey participants were confronted with three MeVO case scenarios that involved patients with an M3, A2, and P2 occlusion, respectively (table 2). Physicians were asked how they would treat the patient: (A) assuming that the patient is eligible for intravenous alteplase; and (B) assuming that the patient is ineligible for intravenous alteplase. Assuming that the patient is eligible for intravenous alteplase, 40% would offer EVT in the A2 and the P2 scenarios, while the EVT rate for the M3 scenarios was lower, at 18% (figure 2). In case of alteplase ineligibility, EVT rates were much higher (A2: 78%, P2: 76%, M3: 59%). These results most likely reflect survey respondents’ reliance on alteplase to open MeVOs, but the fact that most physicians opted for EVT when alteplase was not an option also shows that they consider EVT a safe and effective treatment option for MeVO strokes.
Overview of survey participants. Survey respondents (n=184) were mainly interventional neuroradiologists and practiced in 43 different countries.
Treatment decision of the ESCAPE ALICE participants (n=184) for the three medium vessel occlusion case scenarios. Assuming eligibility for intravenous alteplase (tPA), 18–40% of physicians would offer endovascular treatment (EVT). In the case of ineligibility for intravenous alteplase, 59–78% would proceed with EVT.
Detailed description of medium vessel occlusion case scenarios
Practical challenges when conducting an EVT trial for MeVO strokes
There is no doubt that successfully designing and conducting an EVT trial in MeVO strokes will be challenging, as the clinical deficits caused by MeVOs are generally less severe than in LVO strokes.11 At the same time, the more distal occlusion location likely increases the risk of iatrogenic complications (vessel perforation). In other words, when performing EVT in MeVO strokes, there is less to gain and more to lose compared with LVO strokes. This causes several additional challenges in the field of technology, training and outcome assessment, and has major implications on trial design and execution. Table 3 summarizes the problems that will arise in these three areas and suggests possible solutions to overcome them.
Challenges and possible solutions when conducting an endovascular treatment trial for medium vessel occlusion strokes
Future directions: how to move forward?
Given the poor natural history of MeVO strokes and the increasing evidence for safety and efficacy of EVT in M2 occlusions, an EVT trial in MeVO strokes seems warranted. This however requires several problems to be solved: appropriate devices and techniques that are suitable for MeVO thrombectomy have to be designed and tested, which will take some time. It will also be challenging and take time to develop appropriate outcome measurement tools, and once they are developed, they might not be easily accepted by guideline committees and approval organizations. Thus in the short term, we may remain restricted to already existing and validated scales. Sites and operators for a MeVO trial should be chosen carefully as the margin of benefit is smaller and the risk of complications higher compared with LVO strokes. However, none of these obstacles seem insurmountable. Several small MeVO suitable devices and catheters are already in development. Utilization of simulation technology to improve endovascular training is increasing. Collaborations such as HERMES have opened the doors for conducting worldwide trials within a reasonable period of time even when sample sizes are large. The recently completed ESCAPE-NA1 trial (Clinicaltrials.gov; NCT02930018) enrolled over 1100 patients in 2.5 years. Lessons learnt from previous trials will reduce the likelihood of design and execution errors. We believe that MeVO is the next frontier.
Footnotes
Twitter @johanna_ospel, @mihill68
Correction notice Since the online publication of this article, the sentence 'For medium vessel occlusions (MeVOs)—that is, M2/, A2/3, and P2/3 segment occlusions—the situation is less clear' was updated to 'For medium vessel occlusions (MeVOs)—that is, M2/3, A2/3, and P2/3 segment occlusions—the situation is less clear'
Contributors MG: conceptualization and critical revision of the manuscript. JMO: drafting and critical revision of the manuscript and figures. BKM and MDH: critical revision of the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial. or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.