Article Text
Abstract
Background and aims Platelets and von Willebrand factor (vWF) are key factors in thrombosis and thus are likely key components of acute ischemic stroke (AIS) emboli. We aimed to characterize platelet and vWF levels in AIS emboli and to assess associations between their expression levels and clinical and procedural information.
Materials and method Histopathological and immunohistochemical analysis of emboli collected as part of the multi-institutional RESTORE registry was performed. The composition of the emboli was quantified using Orbit Image Analysis machine learning software. Correlations between clot components and clinical and procedural information were assessed using the χ2 test.
Results Ninety-one emboli samples retrieved from 63 patients were analyzed in the study. The mean platelet (CD42b) content of the clots was 33.9% and the mean vWF content of the clots was 29.8%. There was a positive correlation between platelet and vWF levels (ρ=0.564, p<0.001*, n=91). There was an inverse correlation between both platelets and vWF levels and percentage of red blood cells (RBCs) in the emboli (CD42b vs RBC: ρ=−0.535, p<0.001*, n=91; vWF vs RBC: ρ=−0.366, p<0.001*, n=91). Eighty-one percent of patients in the low platelet group had a good revascularization outcome (Thrombolysis in Cerebral Infarction 2c/3) compared with 58% in the high platelet group (χ2=5.856, p=0.016).
Conclusion Platelet and vWF levels in AIS emboli correlate with each other and both have an inverse relationship with RBC composition. Patients with platelet-rich clots have poorer revascularization outcomes.
- stroke
- platelets
- thrombectomy
Statistics from Altmetric.com
Introduction
There is a growing consensus within the field of acute ischemic stroke (AIS) treatment that the histological structure and composition of the occlusive emboli significantly influences the outcome for patients treated with both recombinant tissue plasminogen activator (rt-PA) and mechanical thrombectomy devices.1–5 Platelets and von Willebrand factor (vWF) are key factors in thrombus formation and have previously been shown to be key components of AIS thromboemboli.6 7
Platelets are anucleated cell fragments that are produced in large numbers by megakaryocytes found mainly in bone marrow.8 They circulate in the blood for 7–10 days and help to maintain homeostasis in healthy non-atherosclerotic vessels. Platelets play a key role in mediating primary hemostasis after endothelial injury.9 Upon vessel wall injury platelets adhere to exposed collagen by means of their collagen receptors GPVI and integrin α2β1, initiating intracellular signaling, further platelet activation, and thrombus formation.10 Under high shear conditions such as in small arteries or stenosis due to atherosclerosis, platelet adhesion becomes more dependent on the interaction between the platelet GPIb receptor and vWF.11
vWF is a large multimeric plasma protein synthesized by megakaryocytes and endothelial cells.12 A significant body of research has implicated vWF as both a key player in thrombus formation and also as a risk factor for AIS.13–17 Elevated serum levels of vWF have been shown to predict increased inpatient complications, neurological worsening, and reduced functional outcomes compared with normal levels.18
Materials and methods
Study design
This General Data Protection Regulation compliant study was approved by the National University of Ireland Galway research ethics committees and the respective institutional research ethics committee at Beaumont Hospital, Dublin, Ireland and Sahlgrenska University Hospital, Gothenburg, Sweden. The inclusion criteria were as follows: patients >18 years, having undergone mechanical thrombectomy treatment for AIS and with embolic material available for analysis. All patients were examined neurologically on arrival and the National Institutes of Health Stroke Scale (NIHSS) score was recorded. Stroke severity on admission was dichotomized using NIHSS scores as mild to moderate (NIHSS <16) and severe (NIHSS ≥16).
Per-pass clot collection and processing
Clots were collected in a per-pass manner, meaning that where multiple procedural passes were used to treat the patients, clot fragments from each pass were collected separately. On retrieval from the patient, each clot was immediately fixed in 10% phosphate-buffered formalin. Clots were shipped to the Department of Physiology in the National University of Ireland Galway and, on arrival, each case and clot fragment was logged in the RESTORE registry. Gross photographs were taken of each clot fragment and all clots were then processed using a standard tissue processing protocol and embedded in paraffin wax. The formalin-fixed paraffin-embedded clot material was cut into 3 µm sections for histology and immunohistochemical staining.
Martius Scarlet Blue staining
Two representative sections were stained with Martius Scarlet Blue (MSB) to identify the standard clot components (red blood cells, white blood cells, fibrin, platelets/other). Briefly, the slides were deparaffinized in xylene and rehydrated through alcohol to water and placed in Bouin's fluid at 56°C in a water bath for 1 hour, then rinsed in running tap water (5 min). They were then placed in filtered iron ammonium-celestine blue solution (10 min), rinsed in running tap water (5 min), stained with filtered Mayer’s hematoxylin (10 min), and rinsed in warm running water to blue. The slides were rinsed with 95% alcohol (1 min) followed by fresh Martius yellow (5 min), rinsed in distilled water (1 min) and then stained in filtered Crystal scarlet (10 min). The slides were then differentiated in fresh phosphotungstic acid (7 min), stained with Methyl blue (10 min), and rinsed in 1% aqueous acetic (1 min). Sections were dehydrated in absolute alcohol (two fast dips), cleared in xylene, and mounted in DPX.
Immunohistochemistry
Immunohistochemical staining for platelets (CD42b) and vWF was performed on a Ventana Discovery autostainer using a Discovery RedMap kit (Roche 760–123). Antigen retrieval with Tris-EDTA was performed for platelet staining (anti-CD42b); no antigen retrieval was used for vWF staining. Primary antibody (anti-CD42b: Abcam ab27669, 1:200 dilution; anti-vWF: Dako A-0082, 1:200 dilution) incubation time was 30 min, which was followed by a 30 min incubation with a universal secondary antibody (Roche 760–4205). Counterstaining of tissue using hematoxylin was performed for 6 min followed by a 2 min incubation in bluing reagent. Sections were then washed in warm soapy water to remove the oil-based liquid coverslip, followed by rinsing in distilled water. Sections were then dehydrated in alcohol, cleared in xylene, and mounted with DPX. Negative controls were performed by omission of the primary antibody step.
Slide scanning and quantification
Histology and immunohistochemically stained slides were scanned on an Olympus vs120 slide scanner at 20× magnification and digital whole-slide scan images were generated. Histologic and immunohistochemical quantification was performed on the digital slides using Orbit Image Analysis Software (www.orbit.bio), as described previously.19 The percentage area of each component (red blood cells (RBCs), white blood cells (WBCs), fibrin, and platelet/other) within the clot was calculated for histological staining with MSB. The percentage area of positive immunohistochemical staining was calculated separately for CD42b and vWF.
Statistical analysis
All statistical correlations were assessed using IBM SPSS Statistics 22. Platelet-rich clots were defined as CD42b ≥mean, vWF-rich clots were defined as vWF ≥mean, etc. Spearman correlations were used to assess associations between continuous variables. Correlations between categorical variables were assessed using the χ2 test. GraphPad Prism 8 was used to generate graphs and figures. The results are reported as mean (SD) or number (%) of cases. The level of statistical significance for all analyses was set at p<0.05.
Results
Patient cohort
Sixty-three patients were included in the study. Table 1 shows the clinical demographics of the patient cohort. Forty-four percent of patients were treated with rt-PA. The suspected etiology was large artery (14.3%), cardioembolic (31.7%), cryptogenic (50.8%), and other (3.2%). Aspiration alone (63.5%) was the most commonly used endovascular treatment strategy, with stent retriever devices being deployed in the remaining 36.5% of patients. Thrombolysis in Cerebral Infarction (TICI) 2c/3 was achieved in 71% of patients treated, with a mean number of passes of 2.4. Fifty-one percent of patients had a severe stroke defined as an NIHSS score of ≥16. The average reported clot length was 15.1 mm. The majority of cases had an internal carotid artery or M1 middle cerebral artery occlusion (28.6% and 68.3%, respectively) and 18 cases (28.6%) had occlusions that spanned two or more locations.
Clinical details of patient cohort
Histological composition
The MSB stain was used to assess the histological composition of the emboli (table 2). Red blood cells were the dominant components of AIS clots with their mean compositions being 39.2%, followed by fibrin (30.7%) and platelets/other (27.7%). The average white blood cell composition was 2.4%. No significant difference in thrombus composition was observed between thrombi that were removed in one pass (54.0%) and thrombi that required multiple passes (46.0%).
Histological and immunohistochemical and Spearman’s rho results
Platelet composition correlates with vWF levels
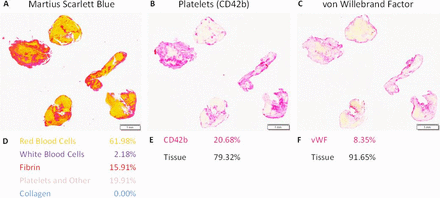
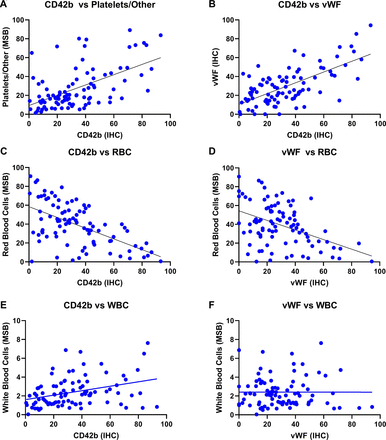
The composition of platelets (CD42b) varied from 0.5% to 93.2% of the total area, with a mean value of 33.9%. There was a positive correlation between platelets quantified using the specific anti-CD42b antibody and platelets/other quantified using the MSB stain (ρ=0.534, p<0.001*, n=91); an example can be seen in figure 1. The composition of vWF varied from 0.1% to 94.3% of the total area, with a mean value of 29.8%. There was a positive correlation between platelet (CD42b) levels and vWF levels (ρ=0.564, p<0.001*, n=91), as can be seen in figure 2.
Sequential 3 µm sections stained with Martius Scarlet Blue (MSB), anti-CD42b (platelets) and anti-von Willebrand factor (anti-vWF). (A) MSB staining demonstrating the presence of red blood cells in yellow, fibrin in red, white blood cells in blue, and platelets and other in grey/pink. (B,C) Immunohistochemical staining with anti-CD42b for platelets and anti-vWF. Sections were stained on a Ventana Discovery Autostainer using a RedMap kit (red=positive, magnification 0.6×). (D, E, F) Quantification results for each of the respective stains (D) MSB, (E) CD42b, and (F) vWF.
Correlations between clot components. (A) A significant positive correlation was observed between platelets (CD42b) measured by immunohistochemistry and platelets and other as measured by Martius Scarlet Blue (MSB) staining (r=0.534**). (B) A significant positive correlation was also observed between platelets (CD42b) and von Willebrand factor (r=0.444**). (C,D) A significant inverse correlation was noted between red blood cells and both platelets (CD42b) (r=−0.535*) and von Willebrand factor (r=0.366**). (E) A significant positive correlation was observed between platelets (CD42b) and white blood cells (r=0.353**). (F) No significant correlation was observed between platelets (CD42b) and fibrin levels.
Inverse correlation between red blood cells and both platelets and vWF
There was an inverse correlation between the percentage of platelets (CD42b) and red blood cell composition (ρ=−0.535, p<0.001*, n=91), as can be seen in table 2. There was also an inverse correlation between the percentage of vWF and red blood cell composition (ρ=−0.366, p<0.001*, n=91).
Positive correlation of platelets with white blood cells
A positive correlation between the percentage of platelets (CD42b) and white blood cell composition was observed (ρ=0.353, p<0.001*, n=91), as shown in table 2. No significant correlations were observed between the level of platelets (CD42b) and fibrin. Similarly, no significant correlations were noted between the level of vWF and fibrin or white blood cells measured using the MSB stain.
Platelet-rich clots are associated with a poorer TICI score
Eighty-one percent of patients in the low platelet group (CD42b <mean) had a good revascularization outcome (TICI 2c/3) compared with 58% in the high platelet group (CD42b >mean) (χ2=5.856, p=0.016). Similarly, 59% of patients in the low platelet cohort had complete revascularization (TICI 3) compared with just 37% in the high platelet cohort (χ2=4.15, p=0.042). vWF levels did not have a significant influence on revascularization outcome. The mechanical thrombectomy technique used (aspiration only vs stent retriever) also did not have a significant influence on final revascularization outcome in any subset of patients.
Clot composition is not associated with stroke severity
The histopathological composition of the clot did not affect stroke severity. None of the clot components quantified including red blood cells, white blood cells, fibrin, platelets, and vWF showed a significant association with stroke severity measured using the NIHSS score (mild to moderate <16; severe ≥16).
Discussion
In this study we demonstrate that platelet-rich clots are associated with poorer revascularization outcomes when mechanical thrombectomy is performed. Additionally, we show that platelet-rich clots are associated with vWF levels, reduced red blood cell content, and increased white blood cell content. These findings are important as they could help to individualize treatment strategy based on clot composition and ultimately improve patient outcome.
In a previous study we demonstrated that platelet-rich clots, as identified using the MSB stain, are isodense on non-contrast CT.20 In that study we quantified red blood cells, white blood cells, fibrin, and platelets/other. We specifically referred to the latter category as platelets/other as we were conscious that the MSB histological stain could not demarcate platelets from other key components of thrombus formation such as vWF and fibrinogen. In this study we use immunohistochemical staining with specific antibodies for both platelets (CD42b) and vWF to quantify the expression levels of each component. We demonstrate that there is a significant positive correlation between platelets/other measured using the MSB stain and platelet levels measured using the platelet-specific anti-CD42b antibody. This finding validates the approach of our previous study and confirms that the MSB stain is a more comprehensive histological stain than the traditional hematoxylin and eosin stain for AIS clot characterization.
Rapid binding of vWF to exposed collagen types I and III after endothelial injury initiates a conformational change in vWF resulting in binding sites for platelet GPIb becoming available.10 The reversible nature of the GPIb–vWF interaction allows for slowing of platelets, thus allowing GPVI and integrin α2β1 to bind to collagen and arrest movement. Subsequent intracellular signaling leads to the synthesis and release of secondary platelet agonists such as thromboxane A2, thrombin, and ADP. These agonists cause platelet surface integrins such as GpIIb/IIIa to shift to a high affinity state.21 Platelet aggregation follows by means of the activated platelet GpIIb/IIIa binding to its primary ligand fibrinogen and also vWF leading to platelet–platelet crosslinking. We show that platelets and vWF account for a considerable proportion of emboli composition with mean values of 33.9% and 29.8%, respectively. Additionally, we show that there is a significant positive correlation between platelets and vWF levels in AIS emboli. It has been suggested the red blood cell-rich clots form as a result of stasis in a vessel, but clots that form under high shear conditions are rich in platelets and vWF.11
The results of our study also demonstrate that there was a significant inverse correlation between both platelets (CD42b), von Willebrand factor levels, and red blood cell composition. These results are in agreement with a recent study on the structural analysis of AIS, which found that red blood cell-rich clots are composed mainly of densely packed red blood cells supported by a thin fibrin network, while platelet-rich clots contain dense fibrin structures aligned with vWF and packed with platelets.22 We found that platelet-rich clots correlated with a poor revascularization outcome and previous studies have also shown that fibrin/platelet-rich clots are more difficult to retrieve than red blood cell-rich clots.23 Contracting (activated) platelets have been shown to actively remodel the fibrin network of thrombi leading to an increase in fibrin density and a consequential decrease in clot volume and increase in clot stiffness.24 This platelet-induced contraction of the fibrin network may help to explain the poorer revascularization outcome in these patients. Further investigation is warranted to understand the initial molecular response to ischemic stroke and how each clot component influences the structural and mechanical properties of the clot and their interaction with mechanical thrombectomy devices.25
It has been suggested that targeting vWF with novel pharmacological agents could help to overcome some of the limitations of current thrombolytic drugs and also in secondary stroke prevention.6 26 It is clear that vWF is a key mediator of thrombus formation and thus is a key component of thromboemboli. However, no significant association was found between stroke severity and vWF composition within the clot. Additionally, none of the other clot components quantified, including platelets, has a significant relationship with stroke severity. This suggests that clot composition is not a key determinant of stroke severity and that occlusion location remains the key determinant of stroke severity.27
Recent evidence suggests that the immune system can have a significant effect on blood coagulation and thrombus formation.28 Atherosclerosis in an inflammatory process resulting in the deposition of cholesterol-rich plaques and under inflammatory conditions the crosstalk between platelets, the coagulation cascade, and the endothelium is no longer able to maintain homeostasis. Platelets and white blood cells appear to have a reciprocal relationship in terms of thrombosis. Activated platelets release cytokines that can modulate white blood cell activation resulting in the formation of white blood cell–platelet aggregates that serve to localize activated white blood cells to the site of the arterial thrombus.29 30 Conversely, activated white blood cells mediate a rapid response to pro-coagulant stimuli by inducing platelet activation and aggregation through the release of platelet activators.31–33 In the current study we demonstrate that there is a significant positive correlation between platelets (CD42b) and white blood cell levels, suggesting that there is a relationship between these two components.
Our study has limitations. First, only clots from patients who had a successful mechanical thrombectomy procedure could be studied. Clots that were not recovered or that dissolved after rt-PA treatment could not be studied. Second, TICI scores were reported at each site and not measured using a central core laboratory, which may have resulted in some site-to-site variability.
Conclusions
Patients with platelet-rich clots have a poorer revascularization outcome. Platelet and vWF levels in AIS emboli correlate with each other and both have an inverse relationship with red blood cell composition. There is a significant positive correlation between platelet (CD42b) and white blood cell levels, suggesting a possible inflammatory mechanism in platelet-rich cases.
Acknowledgments
The authors would like to gratefully acknowledge the invaluable contributions made by the Interventional, Nursing and Clinical coordination teams at each of the sites included in the RESTORE registry. The authors also wish to thank Catherine Curran and our industrial partners Cerenovus.
References
Footnotes
Twitter @FitzSeanT
AD and SF contributed equally.
Contributors AD, SF, and KD were involved in all stages of the manuscript from concept design to drafting the manuscript. LH, MA, MJ, ND, PB, SP, AO’H, EG, IS, TT, AR, and JT were responsible for collecting and recording the clinical and procedural information from patients. All authors reviewed, edited, and approved the final manuscript prior to submission.
Funding This work was supported by the European Regional Development Fund and Science Foundation Ireland (grant number 13/RC/2073).
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon reasonable request.