Summary
Abstract
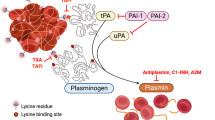
Tranexamic acid is a synthetic derivative of the amino acid lysine that exerts its antifibrinolytic effect through the reversible blockade of lysine binding sites on plasminogen molecules.
Intravenously administered tranexamic acid (most commonly 10 mg/kg followed by infusion of 1 mg/kg/hour) caused reductions relative to placebo of 29 to 54% in postoperative blood losses in patients undergoing cardiac surgery with cardiopulmonary bypass (CPB), with statistically significant reductions in transfusion requirements in some studies. Tranexamic acid had similar efficacy to aprotinin 2 × 106 kallikrein inhibitory units (KIU) and was superior to dipyridamole in the reduction of postoperative blood losses. Transfusion requirements were reduced significantly by 43% with tranexamic acid and by 60% with aprotinin in 1 study. Meta-analysis of 60 trials showed tranexamic acid and aprotinin, unlike ε-aminocaproic acid (EACA) and desmopressin, to reduce significantly the number of patients requiring allogeneic blood transfusions after cardiac surgery with CPB.
Tranexamic acid was associated with reductions relative to placebo in mortality of 5 to 54% in patients with upper gastrointestinal bleeding. Meta-analysis indicated a reduction of 40%.
Reductions of 34 to 57.9% versus placebo or control in mean menstrual blood loss occurred during tranexamic acid therapy in women with menorrhagia; the drug has also been used to good effect in placental bleeding, postpartum haemorrhage and conisation of the cervix. Tranexamic acid significantly reduced mean blood losses after oral surgery in patients with haemophilia and was effective as a mouthwash in dental patients receiving oral anticoagulants.
Reductions in blood loss were also obtained with the use of the drug in patients undergoing orthotopic liver transplantation or transurethral prostatic surgery, and rates of rebleeding were reduced in patients with traumatic hyphaema. Clinical benefit has also been reported with tranexamic acid in patients with hereditary angioneurotic oedema.
Tranexamic acid is well tolerated; nausea and diarrhoea are the most common adverse events. Increased risk of thrombosis with the drug has not been demonstrated in clinical trials.
Conclusions: Tranexamic acid is useful in a wide range of haemorrhagic conditions. The drug reduces postoperative blood losses and transfusion requirements in a number of types of surgery, with potential cost and tolerability advantagesover aprotinin, and appears to reduce rates of mortality and urgent surgery in patients with upper gastrointestinal haemorrhage. Tranexamic acid reduces menstrual blood loss and is a possible alternative to surgery in menorrhagia, and has been used successfully to control bleeding in pregnancy.
Pharmacodynamic Properties
Tranexamic acid exerts its antifibrinolytic effect by blocking lysine binding sites on plasminogen molecules and thereby inhibiting the interaction of plasminogen and the heavy chain of plasmin with lysine residues on the surface of fibrin. Although plasmin can still be formed under these circumstances, it is unable to bind to and degrade fibrin.
Tranexamic acid is 6 to 10 times more potent in terms of binding to plasminogen/plasmin than the other synthetic antifibrinolytic agent ε-aminocaproic acid (EACA). Suppression of fibrinolysis by tranexamic acid is manifested in surgical patients by reductions in blood levels of D-dimer, but the drug has no effect on blood coagulation parameters. Concurrent administration of heparin does not influence the activity of tranexamic acid.
Pharmacokinetic Properties
Maximum plasma concentrations of tranexamic acid are attained within 3 hours of an oral dose; the presence of food in the gastrointestinal tract has no effect on the pharmacokinetic parameters of the drug. Elimination after intravenous administration is triexponential, and over 95% of each dose is eliminated as unchanged drug in the urine. The total cumulative excretion after an intravenous dose is approximately 90% after 24 hours.
Of the total amount of circulating tranexamic acid, 3% is bound to plasminogen. The drug crosses the blood-brain barrier and the placenta, but excretion into breast milk is minimal. Tranexamic acid is not detectable in saliva after systemic (oral) administration, and mouthwashing with 5% w/v aqueous solutions of the drug results in plasma drug concentrations below 2 mg/L.
Therapeutic Use
Cardiac Surgery. Perioperative treatment with tranexamic acid (most commonly as an intravenous loading dose of 10 mg/kg followed by an infusion of 1 mg/kg/hour) resulted in significant reductions in postoperative blood losses (mostly measured over 12 to 24 hours) in randomised, double-blind comparisons with placebo in patients undergoing cardiac surgery with cardiopulmonary bypass (CPB). Losses via mediastinal drains were reduced by 29 to 54% relative to placebo, and statistically significant reductions in red blood cell transfusion requirements were reported in some but not all studies. Inconsistency in results with respect to reduction or elimination of transfusions may have been caused in part by variation between institutions in transfusion criteria.
The protease inhibitor aprotinin has been the most frequently used comparator in randomised but nonblind studies of tranexamic acid in patients undergoing cardiac surgery with CPB. Postoperative blood losses (over 6 hours) were reduced to a similar extent by tranexamic acid 10 mg/kg intravenously followed by infusion of 1 mg/kg/hour and aprotinin 2 × 106 kallikrein inhibitory units (KIU) intravenously in 1 study, with both treatments being superior to dipyridamole. Similar effects on postoperative blood losses with the 2 drugs were reported in 2 further studies. Both agents significantly reduced postoperative transfusion requirements in one of these trials (by 43 and 60% with tranexamic acid and aprotinin, respectively; both p < 0.05 vs control group). Other studies have shown greater reductions in 24-hour blood losses with aprotinin or EACA than with tranexamic acid, but definitive conclusions cannot be drawn from these trials because of inconsistent transfusion data and small patient numbers.
A meta-analysis of 60 randomised clinical trials of haemostatic agents in cardiac surgery with CPB showed tranexamic acid to be associated with a significant decrease (relative to placebo or no treatment) in the proportion of patients requiring allogeneic blood transfusions. A similar effect was found with aprotinin but not with EACA or desmopressin.
Acute Upper Gastrointestinal Bleeding. Reduction of blood transfusion requirements with tranexamic acid therapy in patients with upper gastrointestinal bleeding was first described in 1973. In randomised double-blind studies, predominantly in patients with peptic ulceration or erosion, reductions relative to placebo in mortality rates have ranged from 5 to 54% with tranexamic acid (4.5 to 6g daily for 5 to 7 days in most studies); statistical significance between tranexamic acid and placebo was obtained in the largest published trial.
Meta-analysis of studies of tranexamic acid in patients with upper gastrointestinal haemorrhage showed the drug to be associated with reductions relative to placebo of 20 to 30% in rates of rebleeding, 30 to 40% in the need for surgery and 40% in mortality rates.
Oral Surgery. Proportions of patients with postoperative bleeding complications ranged from 0 to 6.7% when mouthwashes of tranexamic acid were used after oral surgery in patients receiving oral anticoagulant therapy. The corresponding range in patients who received placebo was 13.3 to 40%. In patients with haemophilia, 5 days’ treatment with tranexamic acid 1g 3 times daily orally resulted in a mean blood loss after oral surgery of 61.2ml, compared with an 84.1ml loss with placebo, and reduced consumption of clotting factors (14.3 vs 78.6% of patients).
Other Surgery. Substantial and statistically significant reductions relative to placebo in mean postoperative blood losses (57 and 65.9%) were reported in 2 trials after perioperative tranexamic acid therapy in patients undergoing total knee arthroplasty, with significant reductions in transfusion requirements. Clinical benefit relative to placebo was obtained after intravenous infusion of tranexamic acid 40 mg/kg/hour in 1 study in patients undergoing orthotopic liver transplantation, with no episodes of hepatic artery or portal vein thrombosis occurring within 1 month of surgery.
Four-week incidences of haemorrhage after transurethral prostatic surgery in a randomised study in 100 men were 24% after treatment with tranexamic acid (1g 3 times daily orally) and 56% in patients who received no antifibrinolytic therapy.
Gynaecology. Reductions of 34 to 57.9% versus placebo or control in mean menstrual blood loss were reported in women with menorrhagia receiving 2 to 3 cycles of treatment with tranexamic acid. The drug was at least as effective as nonsteroidal anti-inflammatory therapy and more effective than etamsylate (ethamsylate) or norethisterone. Efficacy of tranexamic acid in the control of bleeding has also been reported in individual patients with placental abruption or postpartum haemorrhage. A mean 71% reduction in postoperative blood loss was noted in a double-blind study in patients who received tranexamic acid 1.5g daily orally for 12 days after conisation of the cervix. In another double-blind study, 1 of 38 patients who received tranexamic acid and 4 of 37 who received placebo experienced late bleeding after cervical conisation with suturing; the difference between groups was not statistically significant.
Other Indications. An oral dosage of tranexamic acid lg 3 times daily significantly reduced the frequency of secondary ocular haemorrhage after traumatic hyphaema in controlled trials; further data from a case series of 340 children showed rates of rebleeding of 1.1% and 9.6% in patients who received tranexamic acid and no antifibrinolytic therapy, respectively.
Reductions versus placebo in number and severity of attacks of oedema in patients with hereditary angioneurotic oedema were reported in 2 randomised, double-blind studies of tranexamic acid, and clinical benefit was obtained with the drug (1.5g orally 3 times daily) in 6 of 7 patients described in a case series.
There was a significant reduction (from 24% with placebo to 9% with tranexamic acid therapy for up to 4 weeks) in the rate of rebleeding in a randomised double blind placebo-controlled study in 479 patients with subarachnoid haemorrhage. However, overall outcome was not improved with tranexamic acid after 3 months; this was attributed to an increase in incidence of cerebral ischaemia.
Tolerability
Tranexamic acid is well tolerated. Adverse events are uncommon and usually manifest as nausea or diarrhoea, or occasionally as orthostatic reactions. Results of controlled clinical studies have not confirmed concerns over the possibility of an increased thrombotic tendency in patients treated with inhibitors of fibrinolysis. No increases in incidence of thrombotic events were reported with tranexamic acid in studies of patients undergoing cardiac surgery with CPB or in a retrospective case analysis of 256 women with bleeding disorders in pregnancy. No muta-genic activity or harmful fetal effects of tranexamic acid have been reported.
Retinal changes seen in dogs after very high dosages of tranexamic acid for 1 year have not been reported in humans receiving the drug at therapeutic dosages. However, disturbances in colour vision have been documented, and patients who develop this symptom should discontinue therapy.
Dosage and Administration
Tranexamic acid is presented in a variety of formulations for oral (tablets and syrup) or intravenous use. A dosage of 500mg to 1g by slow intravenous injection 3 times daily or 1 to 1.5g 2 to 3 times daily orally is recommended for local fibrinolysis. For general fibrinolysis, a single dose of 1g or 10 mg/kg by slow intravenous injection is recommended.
Patients undergoing cardiac surgery have most commonly received tranexamic acid intravenously as a 10 mg/kg dose before CPB and an infusion of 1 mg/kg/hour thereafter. A daily dosage of 4.5 to 6g daily (divided into 3 to 6 doses) for 5 to 7 days (intravenous followed by oral therapy) has been used most frequently in patients with upper gastrointestinal bleeding.
Patients with haemophilia who are about to undergo oral surgery require 1 to 1.5g orally every 8 hours, and a 4.8 to 5% mouthwash, used for 2 minutes 4 times daily for 7 days, has shown good efficacy in dental patients receiving anticoagulant therapy.
Intravenous infusion of 10 mg/kg before release of tourniquet may be used in patients undergoing knee arthroplasty, and treatment with oral tranexamic acid 6 to 12g daily for 4 days has been used in patients undergoing transurethral prostate surgery. Intravenous infusion of 40 mg/kg/hour has been used to good effect in patients undergoing orthotopic liver transplantation.
Women with menorrhagia should receive tranexamic acid 1 to 1.5g 3 to 4 times daily orally for 3 to 4 days. Dosages of 1.5g or 1 to 1.5g orally 3 times daily are recommended for conisation of the cervix or traumatic hyphaema, respectively, and oral treatment with 1.5g 3 times daily is recommended for the management of hereditary angioneurotic oedema.
Tranexamic acid is contraindicated in patients with a history of thromboembolic disease, and dosage reductions are recommended in patients with renal insufficiency.
Similar content being viewed by others
References
Berkow R, Fletcher AJ, editors. The Merck manual of diagnosis and therapy. 15th ed. Rahway (NJ): Merck & Co., 1987
Okamoto S, Sato S, Takada Y, et al. An active stereo isomer (trans-form) of AMCHA and its fibrinolytic (antiplasminic) action in vitro and in vivo. Keio J Med 1964; 13: 177–85
Thorsen S. Differences in the binding to fibrin of native plasminogen and plasminogen modified by proteolytic degradation: influence of ω-aminocarboxylic acids. Biochim Biophys Acta 1975; 393: 55–65
Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the antifibrinolytic action of tranexamic acid. Biochim Biophys Acta 1981 Feb 18; 673: 75–85
Andersson L, Nilsson IM, Niléhn JE, et al. Experimental and clinical studies on AMCA, the antifibrinolytically active isomer of p-aminomethyl cyclohexane carboxylic acid. Scand J Haematol 1965; 2: 230–47
Andersson L, Nilsoon IM, Colleen S, et al. Role of urokinase and tissue activator in sustaining bleeding and the management thereof with EACA and AMCA. Ann N Y Acad Sci 1968; 146: 642–58
Dubber AH, McNicol GP, Douglas AS, et al. Some properties of the antifibrinolytically active isomer of amino-methyl-cyclohexane carboxylic acid. Lancet 1964; II 1317-9
Dubber AH, McNicol GP, Douglas AS. Amino methyl cyclohexane carboxylic acid (AMCHA), a new synthetic fibrinolytic inhibitor. Br J Haematol 1965; 11: 237–45
Castellino FJ. Biochemistry of human plasminogen. Semin Thromb Hemost 1984; 10: 18–23
Sottrup-Jensen L, Claeys H, Zajdel M, et al. The primary structure of human plasminogen: isolation of two lysine-binding fragments and one “mini”-plasminogen(MW, 38,000) by elastase-catalyzed-specific limited proteolysis. Prog Chem Fibrinol Thrombol 1978; 3: 191–209
Anonick PK, Vasudevan J, Gonias SL. Antifibrinolytic activities of α-N-acetyl-L-lysine methyl ester, ε-aminocaproic acid, and tranexamic acid: importance of kringle interactions and active site inhibition. ArteriosclerThromb 1992Jun; 12: 708–16
Horrow JC, Van Riper DF, Strong MD, et al. The dose-response relationship of tranexamic acid. Anesthesiology 1995 Feb; 82: 383–92
Shore-Lesserson L, Reich DL, Vela-Cantos F, et al. Tranexamic acid reduces transfusions and mediastinal drainage in repeat cardiac surgery. Anesth Analg 1996 Jul; 83: 18–26
Blauhut B, Harringer W, Bettelheim P, et al. Comparison of the effects of aprotinin and tranexamic acid on blood loss and related variables after cardiopulmonary bypass. J Thorac Cardiovasc Surg 1994 Dec; 108: 1083–91
Brown RS, Thwaites BK, Mongan PD. Tranexamic acid is effective in decreasing postoperative bleeding and transfusions in primary coronary artery bypass operations: a double-blind, randomized, placebo-controlled trial. Anesth Analg 1997 Nov; 85: 963–70
Ammar T, Silvay G, Reich DL. Platelet effects of aprotinin and tranexamic acid [abstract]. Br J Anaesth 1997 Jun; 78 Suppl. 2: 34
Soslau G, Horrow J, Brodsky I. Effect of tranexamic acid on platelet ADP during extracorporeal circulation. Am J Hematol 1991 Oct;38: 113–9
Dockeray CJ, Sheppard BL, Daly L, et al. The fibrinolytic enzyme system in normal menstruation and excessive uterine bleeding and the effect of tranexamic acid. Eur J Obstet Gynecol Reprod Biol 1987 Apr; 24: 309–18
Benoni G, Lethagen S, Fredin H. The effect of tranexamic acid on local and plasma fibrinolysis during total knee arthroplasty. Thromb Res 1997 Feb 1; 85: 195–206
Boylan J, Sandier A, O’Leary G, et al. Tranexamic acid prophylaxis in liver transplantation: coagulation factor trends [abstract]. Anesthesiology 1994 Sep; 81(3A): A1286
Tsementzis SA, Honan WP, Nightingale S, et al. Fibrinolytic activity after subarachnoid haemorrhage and the effect of tranexamic acid. Acta Neurochir 1990; 103(3–4): 116–21
Van Riper DF, Horrow J, Strong MD, et al. Tranexamic acid is hemostatic when administered only during heparinization [abstract]. Anesthesiology 1993 Sep; 79 Suppl.: abstr. 93
Pilbrant A, Schannong M, Vessman J. Pharmacokinetics and bioavailability of tranexamic acid. Eur J Clin Pharmacol 1981; 20: 65–72
Widlund L, Strömberg S, Hellström H, et al. The disposition of tranexamic acid (AMCA)in various animal species and in man after oral dosage. Stockholm, Sweden: Kabi AB, 1979. (Data on file)
Tovi D, Thulin CA. The ability of tranexamic acid to cross the blood-brain barrier and its use in patients with ruptured intracranial aneurysms. Acta Neurol Scand 1972; 48: 257
Ahlberg A, Eriksson O, Kjellman H. Diffusion of tranexamic acid to the joint. Acta Orthop Scand 1976 Oct; 47: 486–8
Eriksson O, Kjellman H, Nilsson L. Tranexamic acid in human milk after oral administration of Cyklokapron® to lactating women. Stockholm, Sweden: Kabi AB, 1971. (Data on file)
Kullander S, Nilsson IM. Human placental transfer of an anti-fibrinolytic agent(AMCA). Acta Obstet Gynecol Scand 1970; 49: 241–2
Walzman M, Bonnar J. Effects of tranexamic acid on the coagulation and fibrinolytic systems in pregnancy complicated by placental bleeding. Arch Toxicol 1982; 5 Suppl.: 214–20
Sindet-Pedersen S. Distribution of tranexamic acid to plasma and saliva after oral administration and mouth rinsing: a pharmacokinetic study. J Clin Pharmacol 1987 Dec; 27: 1005–8
Guenther CR. Pro: tranexamic acid is better than aprotinin in decreasing bleeding after cardiac surgery. J Cardiothorac Vasc Anesth 1994 Aug; 8: 471–3
Royston D. Blood-sparing drugs: aprotinin, tranexamic acid, and epsilon-aminocaproic acid. Int Anesthesiol Clin 1995 Winter; 33: 155–79
Barrons RW, Jahr JS. A review of post-cardiopulmonary bypass bleeding, aminocaproic acid, tranexamic acid, and aprotinin. Am J Ther 1996 Dec; 3: 821–38
Hardy JF, Bélisle S. Natural and synthetic antifibrinolytics in adult cardiac surgery: efficacy, effectiveness and efficiency. Can J Anaesth 1994 Nov; 41: 1104–12
Laupacis A, Fergusson D, International Study of Peri-operative Transfusion (ISPOT) Investigators. Drugs to minimize peri-operative blood loss in cardiac surgery: meta-analyses using perioperative blood transfusion as the outcome. Anesth Analg 1997 Dec; 85: 1258–67
Hardy J-F, Bélisle S. Natural and synthetic antifibrinolytics: inert, poisonous or therapeutic agents? Can J Anaesth 1997 Sep; 44: 913–5
Coffey A, Pittmam J, Halbrook H, et al. The use of tranexamic acid to reduce postoperative bleeding following cardiac surgery: a double-blind randomized trial. Am Surg 1995 Jul; 61: 566–8
Dryden PJ, O’Connor JP, Jamieson WRE, et al. Tranexamic acid reduces blood loss and transfusion in reoperative cardiac surgery. Can J Anaesth 1997 Sep; 44: 934–41
Hardy JF, Bélisle S, Dupont C, et al. Prophylactic tranexamic acid and ε-aminocaproic acid for primary myocardial revas-cularization. Ann Thorac Surg 1998 Feb; 65: 371–6
Horrow JC, Hlavacek J, Strong MD, et al. Prophylactic tranexamic acid decreases bleeding after cardiac operations. J Thorac Cardiovasc Surg 1990 Jan; 99: 70–4
Horrow JC, Van Riper DF, Strong MD, et al. Hemostatic effects of tranexamic acid and desmopressin during cardiac surgery. Circulation 1991 Nov; 84: 2063–70
Katsaros D, Petricevic M, Snow NJ, et al. Tranexamic acid reduces postbypass blood use: a double-blinded, prospective, randomized study of 210 patients. Ann Thorac Surg 1996 Apr; 61: 1131–5
Corbeau JJ, Monrigal JP, Jacob JP, et al. Comparative effects of aprotinin and tranexamic acid on blood loss in cardiac surgery [in French]. Ann Fr Anesth Reanim 1995; 14(2): 154–61
Menichetti A, Tritapepe L, Ruvolo G, et al. Changes in coagulation patterns, blood loss and blood use after cardiopulmonary bypass: aprotinin vs tranexamic acid vs epsilon aminocaproic acid. J Cardiovasc Surg 1996; 37(4): 401–7
Penta de Peppo A, Pierri MD, Scafuri A, et al. Intraoperative antifibrinolysis and blood-saving techniques in cardiac surgery: prospective trial of 3 antifibrinolytic drugs. Tex Heart Inst J 1995; 22: 231–6
Pugh SC, Wielogorski AK. A comparison of the effects of tranexamic acid and low-dose aprotinin on blood loss and homologous blood usage in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth 1995 Jun; 9: 240–4
Rousou JA, Engelman RM, Flack III JE, et al. Tranexamic acid significantly reduces blood loss associated with coronary revascularization [see comments]. Ann Thorac Surg 1995 Mar; 59: 671–5
Speekenbrink RGH, Vonk ABA, Wildevuur CRH, et al. Hemostatic efficacy of dipyridamole, tranexamic acid, and aprotinin in coronary bypass grafting. Ann Thorac Surg 1995 Feb; 59: 438–42
Karski JM, Teasdale SJ, Norman P, et al. Prevention of bleeding after cardiopulmonary bypass with high-dose tranexamic acid: double-blind, randomized clinical trial. J Thorac Cardiovasc Surg 1995 Sep; 110: 835–42
Cox HT, Poller L, Thomson JM. Evidence for the release of gastric fibrinolytic activity into peripheral blood. Gut 1969; 10: 404–7
Katschinski B, Logan R, Davies J, et al. Prognostic factors in upper gastrointestinal bleeding. Dig Dis Sci 1994 Apr; 39: 706–12
Henry DA, O’Connell DL. Effects of fibrinolytic inhibitors on mortality from upper gastrointestinal haemorrhage. BMJ 1989 Apr 29; 298: 1142–6
Cormack F, Chakrabarti RR, Jouhar AJ, et al. Tranexamic acid in upper gastrointestinal haemorrhage. Lancet 1973 Jun 2; II: 1207–8
Barer D, Ogilvie A, Henry D, et al. Cimetidine and tranexamic acid in the treatment of acute upper-gastrointestinal-tract bleeding. N Engl J Med 1983 Jun 30; 308: 1571–5
Bergqvist D, Dahlgren S, Hessman Y. Local inhibition of the fibrinolytic system in patients with massive upper gastrointestinal hemorrhage. Ups JMed Sci 1980; 85: 173–8
Biggs JC, Hugh TB, Dodds AJ. Tranexamic acid and upper gastrointestinal haemorrhag: a double-blind trial. Gut 1976 Sep; 17: 729–34
Engqvist A, Broström O, von Feilitzen F, et al. Tranexamic acid in massive haemorrhage from the upper gastrointestinal tract: a double-blind study. Scand J Gastroenterol 1979; 14: 839–44
Staël von Holstein CCS, Eriksson SBS, Källén R. Tranexamic acid as an aid to reducing blood transfusion requirements in gastric and duodenal bleeding. BMJ 1987 Jan 3; 294: 7–10
Mannucci PM. Hemostatic drugs. N Engl J Med 1998; 339(4): 245–53
Sindet-Pedersen S, Stenbjerg S. Effect of local antifibrinolytic treatment with tranexamic acid in hemophiliacs undergoing oral surgery. J Oral Maxillofac Surg 1986 Sep; 44: 703–7
Souto JC, Oliver A, Zuazujausoro I, et al. Oral surgery in anti-coagulated patients without reducing the dose of oral anticoagulant: a prospective randomized study. J Oral Maxillofac Surg 1996 Jan; 54: 27–32. discussion 323
Ramström G, Blombäck M. Tooth extractions in hemophiliacs. Int J Oral Surg 1975; 4: 1–17
Borea G, Montebugnoli L, Capuzzi P, et al. Tranexamic acid as a mouthwash in anticoagulant-treated patients undergoing oral surgery: an alternative method to discontinuing anticoagulant therapy. Oral Surg Oral Med Oral Pathol 1993 Jan; 75: 29–31
Ramström G, Sindet-Pedersen S, Hall G, et al. Prevention of postsurgical bleeding in oral surgery using tranexamic acid without dose modification of oral anticoagulants. J Oral Maxillofac Surg 1993 Nov; 51: 1211–6
Sindet-Pedersen S, Ramström G, Bernvil S, et al. Hemostatic effect of tranexamic acid mouthwash in anticoagulant-treated patients undergoing oral surgery. N Engl J Med 1989 Mar 30; 320: 840–3
Forbes CD, Barr RD, Reid G, et al. Tranexamic acid in control of haemorrhage after dental extraction in haemophilia and Christmas disease. BMJ 1972 May 6; 2: 311–3
Klenerman L, Mackie I, Chakrabarti R, et al. Changes in haemostatic system after application of a tourniquet. Lancet 1977; I: 970–2
Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br 1996 May; 78: 434–40
Hiippala ST, Strid LJ, Wennerstrand MI, et al. Tranexamic acid radically decreases blood loss and transfusions associated with total knee arthroplasty. Anesth Analg 1997 Apr; 84: 839–44
Hiippala S, Strid L, Wennerstrand M, et al. Tranexamic acid (Cyklokapron) reduces perioperative blood loss associated with total knee arthroplasty. Br J Anaesth 1995 May; 74: 534–7
Kang Y. Clinical use of synthetic antifibrinolytic agents during liver transplantation. Semin Thromb Hemost 1993; 19: 258–61
Boylan JF, Klinck JR, Sandier AN, et al. Tranexamic acid reduces blood loss, transfusion requirements, and coagulation factor use in primary orthotopic liver transplantation. Anesthesiology 1996 Nov; 85: 1043–8
Kaspar M, Ramsay MAE, Nguyen A-T, et al. Continuous small-dose tranexamic acid reduces fibrinolysis but not transfusion requirements during orthotopic liver transplantation. Anesth Analg 1997 Aug; 85: 281–5
Yassen K, Bellamy MC, Sadek SA, et al. Tranexamic acid reduces blood loss during orthotopic liver transplantation. Clin Transpl 1993 Oct; 7: 453–8
Hedlund PO. Antifibrinolytic therapy with Cyklokapron in connection with prostatectomy. Scand J Urol Nephrol 1969; 3: 177–82
Kaufmann J, Siefker K. Medikamentöse Senkung postoperativer Blutungen nach Prostatektomien (Erfahrungen mit dem Fibrinolysehemmer AMCA). Urologie 1969; 8: 57–9
Gamba G, Fornasari PM, Grignani G, et al. Haemostasis during transvesical prostatic adenomectomy: a controlled trial on the effect of drugs with antifibrinolytic and thrombin-like activities. Blut 1979 Aug; 39: 89–98
Liddell H. Menorrhagia. N Z Med J 1993 Jun 23; 106: 255–7
Higham JM, Shaw RW. Risk-benefit assessment of drugs used for the treatment of menstrual disorders. Drug Saf 1991 May–Jun;6: 183–91
Milsom I, Andersson K, Andersch B, et al. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idio-pathic menorrhagia. Am J Obstet Gynecol 1991 Mar; 164: 879–83
Hallberg L, Nilsson L. Determination of menstrual blood loss. Scand J Clin Lab Invest 1964; 16: 244–8
Bonnar J, Sheppard BL. Treatment of menorrhagia during menstruation: randomised controlled trial of ethamsylate, mefe-namic acid, and tranexamic acid. BMJ 1996 Sep 7; 313: 579–82
Callender ST, Warner GT, Cope E. Treatment of menorrhagia with tranexamic acid: a double-blind trial. BMJ 1970 Oct 24; 4: 214–6
Gleeson NC, Buggy F, Sheppard BL, et al. The effect of tranexamic acid on measured menstrual loss and endometrial fibrinolytic enzymes in dysfunctional uterine bleeding. Acta Obstet Gynecol Scand 1994 Mar; 73: 274–7
Preston JT, Cameron IT, Adams EJ, et al. Comparative study of tranexamic acid and norethisterone in the treatment of ovulatory menorrhagia. Br J Obstet Gynaecol 1995 May; 102: 401–6
Verstraete M. Clinical application of inhibitors of fibrinolysis. Drugs 1985 Mar; 29: 236–61
Sheppard BL, Bonnar J. The ultrastructure of the arterial supply of the human placenta in early and late pregnancy. J Obstet Gynaecol Br Commonw 1974; 81: 497–511
Sheppard BL, Bonnar J. Fibrinolysis in decidual spiral arteries in late pregnancy. Thromb Haemost 1978; 39: 751–7
Svanberg L, Åstedt B, Nilsson IM. Abruptio placentae treatment with the fibrinolytic inhibitor tranexamic acid. Acta Obstet Gynecol Scand 1980; 59: 127–30
As AK, Hagen P, Webb JB. Tranexamic acid in the management of postpartum haemorrhage. Br J Obstet Gynaecol 1996 Dec; 103: 1250–1
Kinn AC. Konisation vid cancer in situ. Läkartidningen 1970; 67: 2529–32
Rybo G, Westerberg H. The effect of tranexamic acid (AMCA) on postoperative bleeding after conization. Acta Obstet Gynecol Scand 1972; 51: 347–50
Landin L-E, Weiner E. Late bleeding after conization: the effect of tranexamic acid (Cyklokapron®). Opusc Med 1975; 20: 280–4
Varnek L, Dalsgaard C, Hansen A, et al. The effect of tranexamic acid on secondary haemorrhage after traumatic hyphaema. Acta Ophthalmol Copenh 1980 Oct; 58: 787–93
Jerndal T, Frisén M. Tranexamic acid (AMCA) and late hyphaema: a double blind study in cataract surgery. Acta Ophthalmol Copenh 1976 Aug; 54: 417–29
Uusitalo RJ, Ranta-Kemppainen L, Tarkkanen A. Management of traumatic hyphema in children. An analysis of 340 cases [see comments]. Arch Ophthalmol 1988 Sep; 106: 1207–9
Morabe ES, Alivia JR, Samonte EP. Comparative study of conservative and tranexamic acid treatment in traumatic hyphema. J Philipp Med Assoc 1992 Jul–Sep; 68: 20–3
Sim TC, Grant JA. Hereditary angioedema: its diagnostic and management perspectives. Am J Med 1990; 88: 656–64
Cugno M, Cicardi M, Agostoni A. Activation of the contact system and fibrinolysis in autoimmune acquired angioedema: a rationale for prophylactic use of tranexamic acid. J Allergy Clin Immunol 1994 May; 93: 870–6
Blohmé G. Treatment of hereditary angioneurotic oedema with tranexamic acid: a random double-blind cross-over study. Acta Med Scand 1972 Oct; 192: 293–8
Sheffer AL, Austen KF, Rosen FS. Tranexamic acid therapy in hereditary angioneurotic edema. N Engl J Med 1972 Aug 31; 287: 452–4
Ohela K. Treatment of hereditary angioneurotic edema with tranexamic acid and cinnarizine. Acta Derm Venereol 1976; 56: 61–7
Loch Macdonald R. Drug treatment of aneurysmal subarachnoid haemorrhage. CNS Drugs 1996 Apr; 5: 264–77
Vermeulen M, Lindsay KW, Murray GD, et al. Antifibrinolytic treatment in subarachnoid hemorrhage. N Engl J Med 1984 Aug 16; 311: 432–7
Nibbelink DW, Torner JC, Henderson WG. Intracranial aneurysms and subarachnoid hemorrhage: a cooperative study. Antifibrinolytic therapy in recent onset subarachnoid hemorrhage. Stroke 1975 Nov–Dec; 6: 622–9
Maurice-Williams RS. Prolonged antifibrinolysis: an effective non-surgical treatment for ruptured intracranial aneurysms? BMJ 1978 Apr 15; 1: 945–7
van Rossum J, Wintzen AR, Endtz LJ, et al. Effect of tranexamic acid on rebleeding after subarachnoid hemorrhage: a double-blind controlled clinical trial. Ann Neurol 1977 Sep; 2: 238–42
Kaste M, Ramsay M. Tranexamic acid in subarachnoid hemorrhage: a double-blind study. Stroke 1979 Sep–Oct; 10: 519–22
Gelmers HJ. Prevention of recurrence of spontaneous subarachnoid haemorrhage by tranexamic acid. Acta Neurochir Wien 1980; 52: 45–50
Fodstad H, Liliequist B, Schannong M, et al. Tranexamic acid in the preoperative management of ruptured intracranial aneurysms. Surg Neurol 1978 Jul; 10: 9–15
Fodstad H, Forssell Å, Liliequist B, et al. Antifibrinolysis with tranexamic acid in aneurysmal subarachnoid hemorrhage: a consecutive controlled clinical trial. Neurosurgery 1981 Feb; 8: 158–65
Chandra B. Treatment of subarachnoid hemorrhage from ruptured intracranial aneurysm with tranexamic acid: a double-blind clinical trial. Ann Neurol 1978 Jun; 3: 502–4
Kassell NF, Torner JC, Adams Jr HP. Antifibrinolytic therapy in the acute period following aneurysmal subarachnoid hemorrhage: preliminary observations from the Cooperative Aneurysm Study. J Neurosurg 1984; 61: 225–30
Fodstad H, Liliequist B. Spontaneous thrombosis of ruptured intracranial aneurysms during treatment with tranexamic acid (AMCA): report of three cases. Acta Neurochir Wien 1979; 49: 129–44
Ry din E, Lundberg PO. Tranexamic acid and intracranial thrombosis [letter]. Lancet 1976 Jul 3; II: 49
Davies D, Howell DA. Tranexamic acid and arterial thrombosis [letter]. Lancet 1977 Jan 1; I: 49
Albronda T, Gökemeyer JDM, van Haeften TW. Transient acute renal failure due to tranexamic acid therapy for diffuse intravascular coagulation. Neth J Med 1991 Aug; 39: 127–8
Fernández Lucas M, Liaño F, Navarro JF, et al. Acute renal failure secondary to antifibrinolytic therapy. Nephron 1995 Apr; 69: 478–9
Robblee J. Graft occlusion following administration of tranexamic acid. Anesth Analg 1995 Apr; 80 Suppl.: SCA141
Lindoff C, Rybo G, Astedt B. Treatment with tranexamic acid during pregnancy, and the risk of thrombo-embolic complications. Thromb Haemost 1993 Aug 2; 70: 238–40
Ekvärn S. Summary and evaluation of toxicological data. Stockholm, Sweden: KabiVitrum AB, 1983. (Data on file)
Theil PL. Ophthalmological examination of patients in long term treatment with tranexamic acid. Acta Ophthalmol Copenh 1981 Apr; 59: 237–41
Pharmacia & Upjohn. Cyklokapron. ABPI Compendium of Data Sheets and Summaries of Product characteristics 1998–1999. London: Datapharm Publications Ltd
Shimada H, Nagai E, Morita H, et al. Mutagenicity studies of tranexamic acid. Oyo Yakuri 1979; 18: 165–72
Melander B, Gleniecki G, Granstrand B, et al. Biochemistry and toxicology of Amikapron®: the antifibrinolytic active isomer of AMCHA. (Acomparative study with epsilon-aminocaproic acid). Acta Pharmacol Toxicol 1965; 22: 340–52
Morita H, Tachizowa H, Akimoto T. Evaluation of the safety of tranexamic acid. Teratogenic effects in mice and rats [in Japanese]. Oyo Yakuri 1971; 5: 415–20
Okajima K, Kohno I, Soe G, et al. Direct evidence for systemic fibrinogenolysis in patients with acquired α2-plasmin inhibitor deficiency. Am J Hematol 1994; 45: 16–24
Tranexamic acid. In: Reynolds JEF, editor. Martindale: the extra pharmacopoiea. 31 st ed. London: Royal Pharmaceutical Society of Great Britain, 1996: 771–2
Murkin JM. Con: tranexamic acid is not better than aprotinin in decreasing bleeding after cardiac surgery. J Cardiothorac Vasc Anesth 1994 Aug; 8: 474–6
Harmon DE. Cost/benefit analysis of pharmacologic hemostasis. Ann Thorac Surg 1996 Feb; 61: S21–5
Luukainen T, Allonen H, Haukkamaa M, et al. Effective contraception with the levonorgestrel-releasing intrauterine device: 12-month report of a European multicenter study. Contraception 1987; 36: 169–79
Weström L. Incidence, prevalence, and trends of acute pelvic inflammatory disease and its consequences in industrialized countries. Am J Obstet Gynecol 1980; 138: 880–92
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: G. Benoni, Department of Orthopaedics, Malmö University Hospital, Malmö, Sweden; D. Bergqvist, Department of Surgery, Uppsala University, Uppsala, Sweden; J. Bonnar, Department of Obstetrics and Gynaecology, Trinity Centre for Health Sciences, St. James’s Hospital, Dublin, Ireland; J.-F. Hardy, Department of Anaesthesia, Montreal Heart Institute, Montreal, Quebec, Canada; P.M. Mannucci, Hemophilia and Thrombosis Centre, Milan, Italy; K. Okajima, Department of Laboratory Medicine, Kumamoto University School of Medicine, Kumamoto, Japan; J.T. Preston, Department of Obstetrics and Gynaecology, Norfolk and Norwich Hospital, Norfolk, England; G. Ramström, Department of Oral and Jaw Diseases, Karolinska Hospital, Stockholm, Sweden.
Data Selection
Sources: Medical literature published in any language since 1966 on tranexamic acid, identified using AdisBase (a proprietary database of Adis International, Auckland, New Zealand),Medline and EMBASE. Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: AdisBase search terms were ‘tranexamic acid’ and ‘haemorrhage’. Medline and EMBASE search terms were ‘tranexamic acid’, ‘pharmacokinetics’, ‘pharmacology’ and ‘therapeutic use’. Searches were last updated 30 Apr 1999.
Selection: Studies in patients undergoing surgery and those with haemorrhagic disorders who received tranexamic acid. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: tranexamic acid, surgery, cardiac, hepatic, orthopaedic, urinary tract, gastrointestinal, haemophilia, gynaecology, haemorrhage, hereditary angioneurotic oedema, hyphaema, subarachnoid, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability, dosage and administration.
Rights and permissions
About this article
Cite this article
Dunn, C.J., Goa, K.L. Tranexamic Acid. Drugs 57, 1005–1032 (1999). https://doi.org/10.2165/00003495-199957060-00017
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199957060-00017