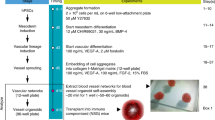
Abstract
The increasing prevalence of diabetes has resulted in a global epidemic1. Diabetes is a major cause of blindness, kidney failure, heart attacks, stroke and amputation of lower limbs. These are often caused by changes in blood vessels, such as the expansion of the basement membrane and a loss of vascular cells2,3,4. Diabetes also impairs the functions of endothelial cells5 and disturbs the communication between endothelial cells and pericytes6. How dysfunction of endothelial cells and/or pericytes leads to diabetic vasculopathy remains largely unknown. Here we report the development of self-organizing three-dimensional human blood vessel organoids from pluripotent stem cells. These human blood vessel organoids contain endothelial cells and pericytes that self-assemble into capillary networks that are enveloped by a basement membrane. Human blood vessel organoids transplanted into mice form a stable, perfused vascular tree, including arteries, arterioles and venules. Exposure of blood vessel organoids to hyperglycaemia and inflammatory cytokines in vitro induces thickening of the vascular basement membrane. Human blood vessels, exposed in vivo to a diabetic milieu in mice, also mimic the microvascular changes found in patients with diabetes. DLL4 and NOTCH3 were identified as key drivers of diabetic vasculopathy in human blood vessels. Therefore, organoids derived from human stem cells faithfully recapitulate the structure and function of human blood vessels and are amenable systems for modelling and identifying the regulators of diabetic vasculopathy, a disease that affects hundreds of millions of patients worldwide.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
WHO. Global Report on Diabetes (WHO, 2016).
Bianchi, E. et al. Age and diabetes related changes of the retinal capillaries: an ultrastructural and immunohistochemical study. Int. J. Immunopathol. Pharmacol. 29, 40–53 (2016).
Roy, S., Ha, J., Trudeau, K. & Beglova, E. Vascular basement membrane thickening in diabetic retinopathy. Curr. Eye Res. 35, 1045–1056 (2010).
Fowler, M. J. Microvascular and macrovascular complications of diabetes. Clin. Diabetes 29, 116–122 (2011).
Eelen, G., de Zeeuw, P., Simons, M. & Carmeliet, P. Endothelial cell metabolism in normal and diseased vasculature. Circ. Res. 116, 1231–1244 (2015).
Warmke, N., Griffin, K. J. & Cubbon, R. M. Pericytes in diabetes-associated vascular disease. J. Diabetes Complicat. 30, 1643–1650 (2016).
Orlova, V. V. et al. Functionality of endothelial cells and pericytes from human pluripotent stem cells demonstrated in cultured vascular plexus and zebrafish xenografts. Arterioscler. Thromb. Vasc. Biol. 34, 177–186 (2014).
Orlova, V. V. et al. Generation, expansion and functional analysis of endothelial cells and pericytes derived from human pluripotent stem cells. Nat. Protoc. 9, 1514–1531 (2014).
Kusuma, S. et al. Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc. Natl Acad. Sci. USA 110, 12601–12606 (2013).
Chan, X. Y. et al. Three-dimensional vascular network assembly from diabetic patient-derived induced pluripotent stem cells. Arterioscler. Thromb. Vasc. Biol. 35, 2677–2685 (2015).
Kusuma, S. & Gerecht, S. Derivation of endothelial cells and pericytes from human pluripotent stem cells. Methods Mol. Biol. 1307, 213–222 (2014).
Cheung, C., Bernardo, A. S., Trotter, M. W. B., Pedersen, R. A. & Sinha, S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat. Biotechnol. 30, 165–173 (2012).
Patsch, C. et al. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat. Cell Biol. 17, 994–1003 (2015).
Ren, X. et al. Engineering pulmonary vasculature in decellularized rat and human lungs. Nat. Biotechnol. 33, 1097–1102 (2015).
James, D. et al. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFβ inhibition is Id1 dependent. Nat. Biotechnol. 28, 161–166 (2010).
Samuel, R., Duda, D. G., Fukumura, D. & Jain, R. K. Vascular diseases await translation of blood vessels engineered from stem cells. Sci. Transl. Med. 7, 309rv6 (2015).
Potente, M., Gerhardt, H. & Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887 (2011).
Samuel, R. et al. Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proc. Natl Acad. Sci. USA 110, 12774–12779 (2013).
Swift, M. R. & Weinstein, B. M. Arterial-venous specification during development. Circ. Res. 104, 576–588 (2009).
Pickup, J. C., Chusney, G. D., Thomas, S. M. & Burt, D. Plasma interleukin-6, tumour necrosis factor α and blood cytokine production in type 2 diabetes. Life Sci. 67, 291–300 (2000).
Wellen, K. E. & Hotamisligil, G. S. Inflammation, stress, and diabetes. J. Clin. Invest. 115, 1111–1119 (2005).
Li, L., Qian, L. & Yu, Z. Q. Serum angiopoietin-2 is associated with angiopathy in type 2 diabetes mellitus. J. Diabetes Complicat. 29, 568–571 (2015).
Lieb, W. et al. Clinical and genetic correlates of circulating angiopoietin-2 and soluble Tie-2 in the community. Circ. Cardiovasc. Genet. 3, 300–306 (2010).
Lim, H. S., Lip, G. Y. H. & Blann, A. D. Angiopoietin-1 and angiopoietin-2 in diabetes mellitus: relationship to VEGF, glycaemic control, endothelial damage/dysfunction and atherosclerosis. Atherosclerosis 180, 113–118 (2005).
Soriguer, F. et al. Apelin levels are increased in morbidly obese subjects with type 2 diabetes mellitus. Obes. Surg. 19, 1574–1580 (2009).
Knudsen, S. T. et al. Increased plasma concentrations of osteoprotegerin in type 2 diabetic patients with microvascular complications. Eur. J. Endocrinol. 149, 39–42 (2003).
Lai, A. K. W. & Lo, A. C. Y. Animal models of diabetic retinopathy: summary and comparison. J. Diabetes Res. 2013, 106594 (2013).
Soler, M. J., Riera, M. & Batlle, D. New experimental models of diabetic nephropathy in mice models of type 2 diabetes: efforts to replicate human nephropathy. Exp. Diabetes Res. 2012, 616313 (2012).
Qaseem, A., Humphrey, L. L., Sweet, D. E., Starkey, M. & Shekelle, P. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 156, 218–231 (2012).
Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 9, 653–660 (2003).
Thomson, J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998).
Bagley, J. A., Reumann, D., Bian, S., Lévi-Strauss, J. & Knoblich, J. A. Fused cerebral organoids model interactions between brain regions. Nat. Methods 14, 743–751 (2017).
Hockemeyer, D. et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat. Biotechnol. 27, 851–857 (2009).
Chen, G. et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 8, 424–429 (2011).
Agu, C. A. et al. Successful generation of human induced pluripotent stem cell lines from blood samples held at room temperature for up to 48 hr. Stem Cell Reports 5, 660–671 (2015).
Ishikawa, F. et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γ chainnull mice. Blood 106, 1565–1573 (2005).
Boxerman, J. L., Schmainda, K. M. & Weisskoff, R. M. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am. J. Neuroradiol. 27, 859–867 (2006).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Varnum-Finney, B. et al. Immobilization of Notch ligand, Delta-1, is required for induction of Notch signaling. J. Cell Sci. 113, 4313–4318 (2000).
Noguera-Troise, I. et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 444, 1032–1037 (2006).
Schoppmann, S. F. et al. Telomerase-immortalized lymphatic and blood vessel endothelial cells are functionally stable and retain their lineage specificity. Microcirculation 11, 261–269 (2004).
Ran, F. A. et al. Genome engineering using the CRISPR–Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Kuleshov, M. V. et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90–W97 (2016).
Chen, E. Y. et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14, 128 (2013).
Haemmerle, M. et al. Enhanced lymph vessel density, remodeling, and inflammation are reflected by gene expression signatures in dermal lymphatic endothelial cells in type 2 diabetes. Diabetes 62, 2509–2529 (2013).
Acknowledgements
We thank all members of our laboratories for constructive critiques and expert advice; N. Fellner (VBCF) for electron microscopy; A. Kavirayani, M. Zeba, T. Engelmaier, J. Klughofer, and A. Piszczek for histology services; G. Michlits and M. Hubmann (U. Elling group) for help with single-guide RNA design and for sharing plasmids; C. Czepe (VBCF) for help with next-generation sequencing experiments; N. A. Calcutt (Department of Pathology, University of California, San Diego) for providing the samples of different rodent models of diabetes; and R. Coward (Department of Nephrology, University of Bristol) for advice on the composition of the diabetogenic medium. The work was supported by the Intramural Research Program of the NHLBI (G.C. and M.B.). J.M.P. is supported by grants from IMBA, the Austrian Ministry of Sciences, the Austrian Academy of Sciences, an ERC Advanced Grant and an Era of Hope Innovator award.
Reviewer information
Nature thanks R. Cubbon, T. Takebe and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
R.A.W. developed the human blood vessel organoids and performed, with A.L., most of the experiments using organoids, including the diabetes in vitro studies and transplantation experiments in mice. N.W., M.H. and B.H., under supervision and with expert advice from D.K., conceived and performed vascular basement membrane analyses on patient samples, human endothelial cells and different rodent models of diabetes and isolated endothelial cells and RNA from patient samples. M.A., under the supervision of J.Z., helped with in vivo transplantations in mice. M.N. performed bioinformatics analysis. F.Y. and B.F performed karyotyping experiments. C.A.A., G.C. and M.B. provided iPS cell lines. C.E., J.A.B. and D.L., under the supervision of J.A.K., generated the reporter embryonic stem cells and C.A.A. helped to characterize the stem cells. J.T. helped with imaging the transplanted vascular organoids. R.A.W. and J.M.P. coordinated the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
A patent application related to this work has been filed. IMBA is in the process of applying for a patent application covering vascular organoid technology that lists R.A.W., D.K. and J.M.P. as inventors.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
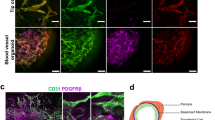
Extended Data Fig. 1 Phenotypical characterization of vascular organoids.
a, Calponin 1+ pericytes are tightly associated with endothelial networks (CD31). Collagen type IV staining is used to visualize the basement membrane. b, Formation of a basement membrane is shown by abluminal collagen type IV deposition. a, b, Experiments were repeated independently n = 10 times with similar results.c, Coculture of differentiated (NC8) endothelial cells and pericytes in a collagen 1:Matrigel matrix. The formed endothelial networks (CD31+), formed only weak interactions with pericytes (PDGFRβ+) and were not enveloped by a collagen type IV+ basement membrane. Experiments were repeated independently n = 3 times with similar results. d, Successful generation of vascular networks from embryonic stem cells (H9) and two independent iPS cell lines. Note how PDGFRβ+ pericytes are in close proximity to the endothelial tubes (CD31+) and the formation of a collagen type IV+ basement membrane. Experiments were repeated independently n = 3 times with similar results. e, Vascular organoid generation from H9 cells. Endothelial networks are shown by CD31 staining and pericytes are shown by PDGFRβ. Experiments were repeated independently n = 10 times with similar results. f, Representative electron microcopy images of vascular organoids (NC8). Note the generation of lumenized, continuous capillary-like structures with the appearance of tight junctions (white arrowheads) and a basement membrane (black arrowheads). Experiments were repeated independently n = 3 times with similar results. g, Tip cells (arrowheads) identified by CD31+ filopodia mark newly forming vessels. The collagen type IV+ basement membrane is absent at the site of active angiogenesis. Experiments were repeated independently n = 10 times with similar results. Scale bars, 2 μm (f), 10 μm (e (right)), 20 μm (g), 50 μm (a, b, d, e (left)), 100 μm (c (right)) and 500 μm (c (left)).
Extended Data Fig. 2 Cellular, molecular and functional characterization of vascular organoids.
a, Left, FACS was used to determine the different cell populations that are present in vascular organoids (NC8). Endothelial cells (ECs) were defined as CD31+VE-cadherin+, pericytes as PDGFRβ+, mesenchymal stem-like cells (MSCs) as CD90+CD73+CD44+ and haematopoietic cells as CD45+. Right, the relative populations of endothelial cells, pericytes, mesenchymal stem-like cells and haematopoietic cells are shown. Data are mean ± s.d. of n = 3 independent experiments. b, Heat map of prototypic marker genes for pluripotent stem cells (PSC), pericytes and endothelial cells. Rows represent genes (log2(TPM + 1)) and columns are samples. FACS-sorted CD31+ endothelial cells and PDGFRβ+ pericytes (P) from vascular organoids (3D iPS cells), from 2D differentiated monocultures (2D iPS cells) or primary 2D monocultures (human umbilical vein endothelial cell (HUVECs) and placental pericytes (Placental-P)) were analysed by RNA-seq and compared to the parental iPS cell line (NC8). Hierarchical clustering of samples demonstrates similar marker gene expression of organoid cells (3D) and primary human cells (HUVECs and placental pericytes). n = 3 biologically independent samples per cell type were analaysed. c, Principal component (PC) analysis was performed on samples from b and separates parental iPS cells (NC8) from differentiated vascular organoid cells (3D). d, Endothelial cells (CD31) in vascular organoids (NC8) are also positive for the lectin UEA-I. Pericytes are visualized by PDGFRβ staining. Experiments were repeated independently n = 10 times with similar results. e, FACS-isolated endothelial cells (CD31+) from vascular organoids take up acetylated low-density lipoprotein (ac-LDL). Experiments were repeated independently n = 3 times with similar results. f, Left, von-Willebrand factor (vWF) expression in endothelial cells (CD31+) from vascular organoids (NC8). Collagen type IV staining is also shown to outline the basement membrane. Right, electron microscopy images show the appearance of Weibel–Palade bodies (white arrowheads). Experiments were repeated independently n = 3 times with similar results. g, TNF-mediated activation of vascular organoids (NC8) revealed by the induction of ICAM-1 expression in endothelial cells (CD31). ICAM-1 induction was determined 24 h after addition of TNF (100 ng ml−1). Experiments were repeated independently n = 3 times with similar results. DAPI was used to counterstain nuclei. Scale bars, 100 nm (f (bottom right)), 500 nm (f (top right)) and 50 μm (d, e, f (left), g).
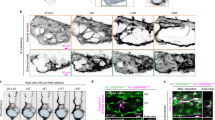
Extended Data Fig. 3 Analysis of vascular organoids transplanted into mice.
a, Intravenous infusion of the human-specific anti-CD31 (hCD31) antibody to label perfused human blood vessels (NC8) transplanted into immunodeficient NSG mice. Mouse vessels are visualized by a mouse-specific anti-CD31 antibody (mCD31). b, Functional human vasculature (detected by human-specific anti-CD31 immunostaining, red) in mice revealed by FITC–dextran perfusion (green). a, b, Experiments were repeated independently on n = 3 biological samples with similar results. c, Representative axial T2-weighted image, blood flow (perfusion), relative blood volume (rBV), mean transit time (MTT) and leakage (K2) measured by MRI. The axial plane was chosen so that both kidneys (outlined in white) and the implant (outlined in red) are visible. The analysed muscle tissue is outlined in green. Quantitative values (mean ± s.d.) for perfusion, relative blood volume, mean transit time and leakage are shown in the table. n = 3 mice. d, Representative low-magnification image of a transplanted vascular organoid stained for E-cadherin and human CD31. e, Arteriole (A) and venule (V) phenotypes appear within the human vascular organoid transplants (NC8). Representative haematoxylin- and eosin-stained histological sections are shown. f, Generation of human arterioles (A) shown by staining for human-specific CD31 (endothelial cells), tightly covered with vascular smooth muscle cells detected by SMA, calponin 1 and human-specific MYH11 immunostaining. As a control, endothelial cells of mouse kidney arterioles do not crossreact with the human-specific CD31 antibody (top right). Samples were also stained with DAPI. g, h, Arterioles of human origin (hCD31+) express arterial markers ephrinB2 and DLL4. i, j, Human venous structures (hCD31+) express the venous markers EPHB4 and COUPTF2. k, BFP-tagged vascular organoids (H9) were transplanted into mice. Staining of the organoids with a human-specific CD31 (hCD31) antibody reveal the human identity of the cells. A representative image from n = 5 mice is shown. d–k, Experiments were repeated independently on n = 3 biological samples with similar results. DAPI was used to counterstain nuclei. Scale bars, 20 μm (e–h), 50 μm (i, j), 100 μm (b, k), 200 μm (a) and 500 μm (d).
Extended Data Fig. 4 Basement membrane changes in diabetic vascular organoids.
a, High glucose, IL-6 and TNF (diabetic) treatment leads to a marked expansion of the collagen type IV+ basement membrane that lines human capillaries (NC8). Calponin 1 immunostaining marks pericytes. Insets show confocal cross-sections of vessel lumina. Right, show 3D reconstructions of basement membrane thickening directly coating the CD31+ endothelial tubes. b, Vascular organoids derived from H9 cells display basement membrane thickening (collagen type IV) upon stimulation with diabetic medium compared to cultures in nondiabetic medium. Endothelial cells were visualized using anti-CD31 immunostaining and pericytes by staining for calponin 1. c, Basement membrane thickening (collagen type IV) in vascular organoids from a second iPS cell line (IPSC2) cultured in diabetic medium. Endothelial cells were visualized using anti-CD31 immunostaining and pericytes were visualised using anti-PDGFRβ immunostaining. a–c, Experiments were repeated independently n = 5 times with similar results. d–f, Increased deposition of the basement membrane components fibronectin (d) laminin (e) and perlecan (f) in diabetic versus nondiabetic vascular organoids (NC8). Endothelial cells were visualized using anti-CD31 immunostaining. Experiments were repeated independently n = 3 times with similar results. g, RT–qPCR of FACS-isolated endothelial cells (CD31+), pericytes (PDGFRβ+) and the remaining negative cells (CD31−PDGFRβ−) from vascular organoids cultured for two days in diabetic or nondiabetic medium. Values are normalized to the endothelial (CD31+) fraction and are shown as mean ± s.e.m. of 3 biological replicates with 50 organoids per experiment. P values were calculated using a two-tailed Student’s t-test. h, Representative electron microscopy images of vascular organoids (NC8), cultured under diabetic and nondiabetic conditions, confirm the marked basement membrane thickening upon diabetic treatment. Note the multiple layers of basement membrane in the diabetic conditions (two-sided arrows) that are not observed in the control organoids (arrowhead). Experiments were repeated independently n = 3 times with similar results. i, Diameter of CD31+ vessels were measured in nondiabetic and diabetic vascular organoids. Values are presented as mean ± s.d.; n = 179 vessels (nondiabetic conditions), n = 151 vessels (diabetic conditions) measured from 4 independent experiments. Scale bars, 5 μm (a (inset), h), 50 μm (a–c) and 100 μm (d–f).
Extended Data Fig. 5 Monocultures of vascular cells in diabetic medium.
a, iPS cell-derived (NC8) endothelial cells cultured in nondiabetic conditions, in 75 mM d-glucose (hyperglycaemia), 1 ng ml−1 TNF and 1 ng ml−1 IL-6 or diabetic medium (hyperglycaemia, TNF and IL-6) were stained for CD31 and collagen type IV. Left, representative images. Right, quantification of collagen type IV intensity of individual endothelial cells. Data are mean ± s.d. of n = 3 independent experiments. P values were calculated using a one-way ANOVA. b, iPS cell-derived (NC8) vascular smooth muscle cells (vSMCs) were cultured in the presence of normal medium, hyperglycaemia, TNF and IL-6 or diabetic medium (hyperglycaemia, TNF and IL-6). Cultures were stained for SMA, collagen type IV and fibronectin (FN). Collagen type IV and fibronectin intensity of individual cells was quantified. Data are mean ± s.d. of n = 3 independent experiments. P values were calculated using a one-way ANOVA. c, G1S1 endothelial cells were cultured in nondiabetic normal medium and diabetic medium (hyperglycaemia, TNF and IL-6) and stained for fibronectin, laminin or collagen type IV. Phalloidin was used to visualize the actin cytoskeleton. Experiments were repeated independently n = 3 times with similar results. d, COL4A1 expression was measured by RT–qPCR from G1S1 cells cultured in nondiabetic and diabetic media for different time points, as indicated. Expression was normalized to HPRT expression. n = 3 independent experiments. P values were calculated using a two-tailed Student’s t-test. Scale bars, 100 μm (a–c).
Extended Data Fig. 6 Analysis of diabetic vascular organoids.
a, b, FACS analysis of vascular organoids (NC8) cultured in nondiabetic or diabetic media. a, Representative FACS plots of endothelial cells (CD31+) and pericytes (PDGFRβ+) from diabetic and nondiabetic control vascular organoids. Experiments were repeated independently n = 6 times with similar results. b, Quantification of numbers of CD31+ endothelial cells, PDGFRβ+ pericytes and CD31−PDGFRβ− cells in diabetic and nondiabetic organoids. Data are mean ± s.e.m. of n = 6 independent experiments. P values were calculated using a two-tailed Student’s t-test. c,d, Pericyte (PDGFRβ) association with endothelial networks (CD31) in vascular organoids is shown for nondiabetic and diabetic conditions in representative images. Note in c the increase in collagen type IV deposition around the endothelium under diabetic conditions. Experiments were repeated independently n = 5 times with similar results. e, Transcriptome analysis of CD31+ endothelial cells (iPS cell-derived endothelial cells (iPSC-ECs)), FACS sorted from vascular organoids (NC8) cultured under diabetic (hyperglycaemia, IL-6 and TNF) and nondiabetic control conditions. Heat maps of differentially expressed genes and the top 5 upregulated genes (ranked by P value) are shown for two independent organoids cultures for each condition. f, GO biological processes of upregulated genes are shown for iPS cell-derived endothelial cells, FACS sorted from vascular organoids (NC8) cultured under diabetic and nondiabetic control conditions. g, GO molecular function terms comparing upregulated genes from CD31+ iPS cell-derived endothelial cells from diabetic blood vessel organoids and T2D patient-derived dermal endothelial CD31+ cells (patient-derived endothelial cells) are plotted with their respective P value. Upregulated genes in patients were derived from sorted CD31+ endothelial cells from patients with T2D compared to those from nondiabetic individuals. f, g, n = 2 biologically independent iPS cell-derived endothelial cell samples per group, n = 4 independent patients per group were sequenced. P values were calculated on differentially expressed genes using Enrichr software (Fisher exact test).
Extended Data Fig. 7 Diabetic vascular basement membrane in vivo and treatment of diabetic vascular organoids with drugs that are commonly used to treat diabetes.
a, Electron microscopy of transplants isolated from control normoglycaemic or diabetic mice. Note the increased thickness and density of collagen fibrils (triangles) around the human vessels of the diabetic mice. Triangles indicate the basement membrane. Experiments were repeated independently on n = 4 biological samples with similar results. b, Collagen type IV staining of the mouse kidney (adjacent to the human transplant) reveals no basement membrane thickening of endogenous vessels at 10 weeks after STZ induction (diabetic). Note the lack of crossreactivity of the human-specific CD31 antibody with the renal mouse endothelium. Experiments were repeated independently on n = 3 biological samples with similar results. c, Quantification of the basement membrane thickness of dermal blood capillaries in the indicated rat and mouse models of diabetes compared to nondiabetic control cohorts. See Supplementary Table 2 for details. Data are mean ± s.d. of analysed blood vessels. ob/ob, db/db mice, n = 5; insulin receptor knockout (IRko) mice, n = 7; STZ-treated mice (17 weeks), n = 3; STZ-treated mice (24 weeks), n = 5; Zucker diabetic fatty (ZDF) rats, n = 3. Age-matched C57BL/KsJ and C57BL/Ks wild-type mice were used as controls. For the ZDF rat model, heterozygous rats (fa/+) were used as controls. Basement membrane thickening was determined based on morphometric analyses of collagen type IV immunostaining. d, Representative images of skin sections of various mouse models of diabetes and respective controls stained for collagen type IV to visualize the basement membrane and CD31 to visualize endothelial cells. Experiments were repeated independently on n = 5 biological samples with similar results. e, Human blood vessel organoids (NC8) were cultured in vitro under diabetic conditions and treated with commonly prescribed diabetic drugs. The changes in deposition of vascular basement membranes were analysed by collagen type IV stainings. Insets show confocal cross-sections of luminal vessels covered by collagen type IV. Experiments were repeated independently n = 3 times with similar results. f, Optical cross-sections of collagen type IV were used to quantify basement membrane thickening. Each lumenized vessel is shown as a dot. Data are mean ± s.d. The indicated number of individual nondiabetic (n = 142), vehicle (n = 124), thiazolidinedione (n = 156), metformin (n = 137), acarbose (n = 152), glimepiride (n = 136), diphenyleneiodonium (n = 141), nateglindine (n = 152) and pioglitazone (n = 142) vessels were analysed for each experimental condition from 3 independent biological replicates. ***P < 0.0001; one-way ANOVA. Drug doses and culture conditions are described in the Methods. Scale bars, 2 μm (a (bottom)), 10 μm (a (top), e (inset)), 20 μm (e) and 50 μm (b, d).
Extended Data Fig. 8 DAPT treatment of diabetic vascular organoids.
a, Representative images of vascular organoids cultured in diabetic medium in the presence of vehicle or the indicated doses of the γ-secretase inhibitor DAPT. Endothelial networks are shown using CD31 staining and the basement membrane is visualized by collagen type IV staining. Experiments were repeated independently n = 3 times with similar results. b, Quantification shows collagen type IV thickness of nondiabetic (n = 136), vehicle (n = 126), 12.5 μM DAPT (n = 208), 25 μM DAPT (n = 142) and 50 μM DAPT (n = 96) vessels (dots) from 5 organoids (NC8) per condition exposed to vehicle or different doses of DAPT. Data are mean ± s.d. ***P < 0.0001; one-way ANOVA. c, d, Proliferation of endothelial cells (CD31) and pericytes (PDGFRβ) in diabetic and DAPT-treated vascular organoids. c, The numbers of endothelial cells (CD31), pericytes (PDGFRβ) and KI-67+ proliferating cells were analysed in vascular organoids treated with indicated treatments. Experiments were repeated independently n = 3 times with similar results. d, Quantification of KI-67+ (proliferative) endothelial cells (CD31+), pericytes (PDGFRβ+) and CD31−PDGFRβ− cells in vascular organoids under diabetic and DAPT-treated conditions. Data are mean ± s.d. of n = 4 (vehicle, DAPT), n = 5 (nondiabetic) vascular organoids. P values were calculated using a one-way ANOVA. e, Heat map of prototypic marker genes for pluripotent stem cells (PSCs), pericytes and endothelial cells. Rows represent genes (log2(TPM + 1)) and columns are samples. FACS-sorted CD31+ endothelial cells and PDGFRβ+ pericytes (P) from nondiabetic (Control), diabetic (Diabetic) or DAPT-treated diabetic (+DAPT) vascular organoids (3D iPS cells), from 2D differentiated monocultures (2D iPS cells) or primary 2D monocultures (HUVECs and placental pericytes) were analysed by RNA-seq and compared to the parental iPS cell line (NC8). Hierarchical clustering of samples shows similar marker gene expression of human primary endothelial cells (HUVECs) and pericytes (placental pericytes) and organoid endothelial cells and pericytes cultured under nondiabetic or diabetic conditions in the absence or presence of the γ-secretase inhibitor DAPT. f, Principal component analysis was performed on samples from e. The close clustering of untreated and diabetic endothelial cells and pericytes in the absence or presence of DAPT shows that cells within the vascular organoids maintain their differentiated cell fate. e, f, n = 3 biologically independent samples per cell type and treatment were analysed. Scale bars, 5 μm (a (inset)) and 50 μm (a, c).
Extended Data Fig. 9 DAPT treatment of vascular organoids in vivo and DLL4 and NOTCH3 knockout vascular organoids.
a, b, Analysis of vascular organoids transplanted into nondiabetic or diabetic STZ-treated mice treated with vehicle or DAPT. a, Human blood vessels were transplanted into diabetic STZ-treated mice treated with or without DAPT and were stained for expression of the human-specific endothelial markers VE-cadherin, CD34 (hCD34) and von-Willebrand factor (hvWF). Note the absence of signal in the adjacent mouse kidney. b, Human blood vessels were transplanted into diabetic STZ-treated mice treated with or without DAPT and were stained for the pericyte-specific markers PDGFRβ, NG2, SM22 and SMA. Costaining with a human-specific CD31 (hCD31) antibody was used to identify human blood vessels. a, b, Experiments were repeated independently on n = 3 biological samples with similar results. c, d, CRISPR-Cas9 genome editing was used to generate DLL4 and NOTCH3 knockout iPS cells (NC8). sgRNAs are indicated in the NOTCH3 and DLL4 sequences as well as the generated indels. c, Western blot shows ablation of NOTCH3 expression in target iPS cells. Clone 4 (red) was used for functional assays. FL, full-length NOTCH3; NTM, transmembrane NOTCH3 subunit. d, Immunostaining in control vascular organoids shows expression of DLL4 in endothelial cells (CD31+) but not in CRISPR-Cas9 genome-edited iPS cells. c, d, Experiments were repeated independently n = 2 times with similar results. e, Vascular organoids differentiated from DLL4 knockout (KO) and NOTCH3 knockout iPS cells (NC8) were stained for markers of endothelial cells (CD31) and pericytes (PDGFRβ). Experiments were repeated independently n = 3 times with similar results. f, Quantification of endothelial networks (CD31+ area) in DLL4 knockout and NOTCH3 knockout vascular organoids. Data are mean ± s.d. from n = 3 independent experiments. P values were calculated using a one-way ANOVA. g, Quantification of pericyte number in DLL4 knockout and NOTCH3 knockout vascular organoids. Data are mean ± s.d. from n = 3 independent experiments. P values were calculated using a one-way ANOVA. h, Representative images of basement membranes stained for collagen type IV from control, DLL4 knockout and NOTCH3 knockout vascular organoids (NC8 iPS cells) exposed to hyperglycaemia, IL-6 and TNF (diabetic) or maintained under standard culture conditions (nondiabetic). Experiments were repeated independently n = 3 times with similar results. i, Thickness of continuously surrounded lumina by collagen type IV was measured in optical cross-sections. Each individual measurement from a lumenized vessel is shown as a dot. A total of nondiabetic (control (n = 265), DLL4 knockout (n = 203) and NOTCH3 knockout (n = 215)) and diabetic (control (n = 214), DLL4 knockout (n = 187), NOTCH3 knockout (n = 206)) lumina were analysed for each experimental condition from 3 independent biological replicates with equal sample size. Data are mean ± s.d. ***P < 0.001; one-way ANOVA. Scale bars, 20 μm (a, b, e) and 50 μm (d, h).
Extended Data Fig. 10 NOTCH3 expression and signalling in vascular organoids.
a, Vascular organoids were cultured under nondiabetic or diabetic conditions and costained for NOTCH3 expression and markers of endothelial cells (CD31) and pericytes (PDGFRβ). Predominant localization of NOTCH3 in pericytes is indicated by the white arrowheads. Experiments were repeated independently n = 3 times with similar results.b, RT–qPCR of endothelial cells (CD31+), pericytes (PDGFRβ+) and the remaining CD31−PDGFRβ− cells shows the highest expression of NOTCH3 in pericytes. Data are mean ± s.e.m. of 3 independent experiments. c–h, Human vascular organoids transplanted into nondiabetic control or diabetic STZ-treated mice were analysed for NOTCH3 expression and the Notch downstream targets HES5 and HEY1. c, e, g, Histological sections of transplanted human blood vessels were stained for NOTCH3 (c), HES5 (e) or HEY1 (g) and costained for SMA (pericytes) and hCD31 (human endothelium). Note the localization of NOTCH3, HES5 as well as HEY1 in SMA+ pericytes. Experiments were repeated independently on n = 3 biological samples with similar results. d, f, h, Quantification of NOTCH3 (d), HES5 (f) and HEY1 (h) expression. Pericytes were segmented using SMA and the intensity of immunofluorescence signal for each marker was measured. Data are mean ± s.e.m. of n = 3 mice. The P value was calculated using a two-tailed Student’s t-test. i, Diabetic mice transplanted with human vascular organoids (H9 ES cells) were treated with a NOTCH3-blocking antibody. Basement membrane thickness of individual human blood vessels (hCD31+) of nondiabetic (n = 223), diabetic and vehicle-treated (n = 212), and diabetic and anti-NOTCH3 antibody-treated (n = 143) mice was determined based on collagen type IV staining. n = 3 nondiabetic and diabetic and vehicle-treated mice; n = 2 diabetic, anti-NOTCH3 antibody-treated mice. Data are mean ± s.d. Scale bars, 50 μm (a, c, e, g, i).
Supplementary information
Supplementary Information
This file contains Supplementary Figure 1 (Genomic integrity of the iPSC line NC8); Supplementary Figure 2 (Genomic integrity of the iPSC#2 and iPSC#3 lines); Supplementary Figure 3 (Flow cytometry gating strategies); uncropped gels; Supplementary Table 1 (Comparison of patient characteristics and laboratory parameters between patients with and without type 2 diabetes (T2D)); and Supplementary Table 2 (Overview of rodent models analysed).
Supplementary Video 1
Confocal stack of vascular organoid stained for CD31. Dimensions: x=y=1417.0μm; stack z= 875.3μm is shown at 15 frames per second.
Supplementary Video 2
Confocal stack of vascular organoid stained for CD31 (red) and PDGFR-β (green). Dimensions: x=y=708.5μm; stack z= 383.4μm is shown at 13.6 frames per second.
Rights and permissions
About this article
Cite this article
Wimmer, R.A., Leopoldi, A., Aichinger, M. et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 565, 505–510 (2019). https://doi.org/10.1038/s41586-018-0858-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-018-0858-8
This article is cited by
-
Advances in secondary prevention mechanisms of macrovascular complications in type 2 diabetes mellitus patients: a comprehensive review
European Journal of Medical Research (2024)
-
Pituitary stem cells: past, present and future perspectives
Nature Reviews Endocrinology (2024)
-
Generating human bone marrow organoids for disease modeling and drug discovery
Nature Protocols (2024)
-
A microfluidic platform integrating functional vascularized organoids-on-chip
Nature Communications (2024)
-
Using stem cells to model the human bone marrow in a dish
Nature Methods (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.