-
PDF
- Split View
-
Views
-
Cite
Cite
Brent M. Egan, Jan N. Basile, Shakaib U. Rehman, Phillip B. Davis, Curt H. Grob, Jessica Flynn Riehle, Christine A. Walters, Daniel T. Lackland, Carmen Merali, Jean E. Sealey, John H. Laragh, Plasma Renin Test–Guided Drug Treatment Algorithm for Correcting Patients With Treated but Uncontrolled Hypertension: A Randomized Controlled Trial, American Journal of Hypertension, Volume 22, Issue 7, July 2009, Pages 792–801, https://doi.org/10.1038/ajh.2009.63
Close - Share Icon Share
Abstract
Undefined pathophysiologic mechanisms likely contribute to unsuccessful antihypertensive drug therapy. The renin test–guided therapeutic (RTGT) algorithm is based on the concept that, irrespective of current drug treatments, subnormal plasma renin activity (PRA) (<0.65 ng/ml/h) indicates sodium-volume excess “V” hypertension, whereas values ≥0.65 indicate renin–angiotensin vasoconstriction excess “R” hypertension.
The RTGT algorithm was applied to treated, uncontrolled hypertensives and compared to clinical hypertension specialists' care (CHSC) without access to PRA. RTGT protocol: “V” patients received natriuretic anti-“V” drugs (diuretics, spironolactone, calcium antagonists, or α1-blockers) while withdrawing antirenin “R” drugs (converting enzyme inhibitors, angiotensin receptor antagonists, or β-blockers). Converse strategies were applied to “R” patients. Eighty-four ambulatory hypertensives were randomized and 77 qualified for the intention-to-treat analysis including 38 in RTGT (63.9 ± 1.8 years; baseline blood pressure (BP) 157.0 ± 2.6/87.1 ± 2.0 mm Hg; PRA 5.8 ± 1.6; 3.1 ± 0.3 antihypertensive drugs) and 39 in CHSC (58.0 ± 2.0 years; BP 153.6 ± 2.3/91.9 ± 2.0; PRA 4.6 ± 1.1; 2.7 ± 0.2 drugs).
BP was controlled in 28/38 (74% (RTGT)) vs. 23/39 (59% (CHSC)), P = 0.17, falling to 127.9 ± 2.3/73.1 ± 1.8 vs. 134.0 ± 2.8/79.8 ± 1.9 mm Hg, respectively. Systolic BP (SBP) fell more with RTGT (−29.1 ± 3.2 vs. −19.2 ± 3.2 mm Hg, P = 0.03), whereas diastolic BP (DBP) declined similarly (P = 0.32). Although final antihypertensive drug numbers were similar (3.1 ± 0.2 (RTGT) vs. 3.0 ± 0.3 (CHSC), P = 0.73) in “V” patients, 60% (RTGT) vs. 11% (CHSC) of “R” drugs were withdrawn and BP medications were reduced (−0.5 ± 0.3 vs. +0.7 ± 0.3, P = 0.01).
In treated but uncontrolled hypertension, RTGT improves control and lowers BP equally well or better than CHSC, indicating that RTGT provides a reasonable strategy for correcting treated but uncontrolled hypertension.
Hypertension afflicts about a billion people worldwide and the majority is uncontrolled.1 In the United States alone, roughly 37 million are uncontrolled including ~17 million receiving drug treatment.2,3 Several strategies have been advanced to improve their blood pressure (BP) control. Stepped-care, with progressive addition of medications but no subtractions, is the most widely used. With this strategy, only 66% were controlled to goal in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial with an average of two medications and 13% required four or more.4 Other treatment strategies are based on age and race with the assumption that Caucasians <55 years old have renin-dependent hypertension and that other racial ethnic groups and individuals ≥55 years old are predominantly low renin.5 Yet other strategies include noninvasive hemodynamic testing, selected genetic polymorphisms, plasma renin activity (PRA) and/or aldosterone testing, and adding an aldosterone antagonist to uncontrolled patients.6–14 Given the public health burden of uncontrolled hypertension, strategies are needed that are broadly applicable in clinical settings. National guidelines advocate referral to clinical hypertension specialists15 and a strategy has been described for leveraging the impact of these specialists.16 Nonetheless, their number is quite insufficient to manage the 17 million treated but uncontrolled hypertensive patients in the United States.
In one study, renin test–guided therapeutics (RTGTs), using ambulatory PRA values obtained from a routine blood sample regardless of antihypertensive therapy or diet, led to clinically significant BP reductions while decreasing the number of antihypertensive medications.13 Therefore, in treated but uncontrolled hypertensive patients, we decided to compare the effectiveness of RTGT17,18 to clinical hypertension specialists' care (CHSC). In the RTGT algorithm, hypertensive patients with low ambulatory PRA values <0.65 ng/ml/h are presumed to have salt-volume excess or “V” hypertension that responds primarily to natriuretic antivolume “V”-type drugs, i.e., aldosterone antagonists, sulfonamide diuretics, calcium channel blockers (CCBs), or α1-antagonists18–23 but not to antirenin–angiotensin system “R” drug types, i.e., β-blockers, angiotensin-converting enzyme inhibitors (ACEIs), or angiotensin receptor blockers.24 Conversely, hypertensive patients with ambulatory PRA ≥0.65 ng/ml/h are considered to be progressively more likely to have renin–angiotensin system-dependent vasoconstriction hypertension and to respond preferentially to “R”-type drugs.18,25–30 Moreover, for the treated but uncontrolled patients who were the subjects of this trial, “V” drugs were stopped in “R” patients with high (>6.5 ng/ml/h) PRA levels, as large reactive increases in renin secretion can oppose antihypertensive drug efficacy.24 Similarly, “R” drugs were stopped in “V” patients because of their limited efficacy24 and because β-adrenergic blockers can raise their BP.31 CHSC physicians performed both limbs of the trial but did not have access to PRA values for treating CHSC patients.
Methods
Patients. Adults ≥21 years of age with treated, uncontrolled hypertension were recruited from the Hypertension Clinics at the Ralph H. Johnson Veterans Affairs Hospital (J.N.B., S.U.R.) and the Medical University of South Carolina (B.M.E.). Patients read and signed an informed consent document approved by the Office of Research Protection at the Medical University of South Carolina.
Study design. This study is a randomized, unblinded (“open label”) trial comparing plasma RTGTs to CHSC in treated but uncontrolled hypertension.
Inclusion criteria. Patients who were referred for uncontrolled hypertension and established clinic patients who had uncontrolled hypertension and who were taking one or more antihypertensive drug types were eligible. To qualify for this study, these individuals were also required to have uncontrolled hypertension as defined by the mean of three quietly seated clinic readings at the single baseline visit ≥140/≥90 mm Hg, except for those with diabetes mellitus and/or nephropathy for whom readings ≥130/≥80 qualified.
Exclusion criteria. Uncontrolled diabetes or hyperlipidemia requiring medication changes; any active disease process requiring new diagnostic and therapeutic plans; any life-threatening illness; history of alcohol or drug abuse in the past 5 years; mental illness or personality disorder that might interfere with adherence to study protocol; end-stage renal disease and progressive chronic kidney disease with serum creatinine >2.5 mg/dl; intolerance to two or more classes of antihypertensive medications; normal home BP <135/85 mm Hg at baseline for patients without diabetes or chronic kidney disease (estimated glomerular filtration rate <60 ml/min/1.73 m2)32 and <125/75 for patients with diabetes or chronic kidney disease,15 i.e., office hypertension, a common measurement artifact in patients with treated and uncontrolled hypertension.33
Patient randomization. After obtaining informed consent, the physician received the randomized assignment from the Data Coordinating Center. The randomization was balanced, i.e., 1:1, and not stratified for any demographic or clinical variables. The first patient was randomized 30 October 2003. The last study visit was completed 11 September 2005.
Baseline assessment. A history and physical examination were performed at the initial visit. A sample for PRA was obtained at the initial visit irrespective of the fasting status. A complete metabolic panel, lipid profile, and a urinalysis with albumin:creatinine were obtained at the time of the initial visit if the patient had fasted overnight and at a separate visit fasting if in the fed state at the initial visit. A complete blood count, thyrotropic hormone level, and an electrocardiogram were obtained if these tests had not been done in the previous year.
Measurements.
Clinic BP: BP readings with the Omron (HEM-712C, 712CLC; Omron Healthcare, Vernon Hill, IL) were obtained with an appropriately sized arm cuff in triplicate after 5 min in the quietly seated position by study personnel. The mean of the three readings was used to determine systolic BP (SBP) and diastolic BP (DBP) at each study visit.
Home BP: All subjects were trained in the use of and given an Omron kit identical to that described for clinic BP measurements. They recorded BP twice daily in the week prior to each visit.
PRA assay: Blood was drawn into a 7 ml EDTA Vacutainer tube in quietly seated, but otherwise ambulatory patients. Blood was processed at room temperature. Plasma was frozen to avoid inadvertent conversion of prorenin to active renin. Frozen plasmas were express mailed to New York. PRA was measured by an enzyme kinetic radioimmunoassay identical to that used by Quest Diagnostics, which involves a 3-h Ang I generation step followed by radioimmunoassay of the formed Ang I.34 Samples with PRA <1.0 ng/ml/h were repeated using an 18-h Ang I generation step. PRA results were e-mailed to the coordinating center within 2–5 days.
Protocol.
RTGT: PRA was used to determine whether uncontrolled hypertension was due to either (i) a persistent body salt excess as indicated by suppressed ambulatory PRA (<0.65 ng/ml/h) or (ii) an excess of plasma renin vasoconstrictor activity (PRA ≥0.65 ng/ml/h). As indicated in Table 1,18 the PRA level guided addition and/or subtraction of “V” or “R” drugs in the RTGT group. The PRA values in Table 1 of <0.65, 0.65–6.5, and >6.5 are the prespecified values for the low-, medium-, and high-renin groups, respectively. “R” drugs were not withdrawn in patients with compelling indications.15 BP outcomes with RTGT were compared to CHSC, in which PRA was not available to guide medication changes.
Renin test–guided therapeutics (RTGT) algorithm for patients unsuccessfully treated on one or more antihypertensive medications18 (No Δ = no change in drug regimen)
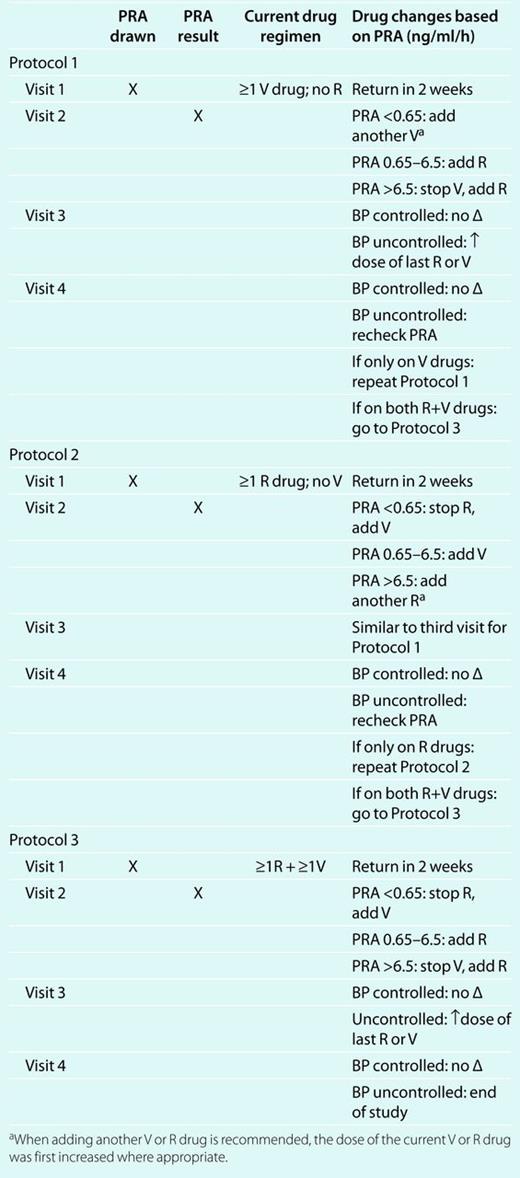 |
 |
Renin test–guided therapeutics (RTGT) algorithm for patients unsuccessfully treated on one or more antihypertensive medications18 (No Δ = no change in drug regimen)
 |
 |
Patient management. Patients were managed by one of three clinical hypertension specialists (B.M.E., J.N.B., S.U.R.). Patients were scheduled every 2–4 weeks until BP was controlled or until the clinician determined that appropriate therapeutic adjustments had been made. Medication adherence was not formally verified, although the three clinical hypertension specialists routinely request that patients bring all medication bottles to each visit where the prescription date and remaining refills are checked for conformity with the visit date. Patients received lifestyle recommendations generally accorded to all patients.
Data management. Study data were single-entered into a Microsoft Access database and verified by two individuals who together compared the data entries to the source documents.
Sample-size estimates. The study was powered on the primary outcome of a difference in BPs between RTGT and CHSC using two-sample t-tests. A minimum of 60 patients with treated and uncontrolled hypertension was determined as the sample size required for 90% power (β = 0.10) to detect a 4 mm Hg (±8 mm Hg variance) difference in the change of both SBP and DBP from baseline to final visit at α = 0.01 between RTGT and CHSC.
Data reporting and analyses. The Access database was converted to a Statistical Analysis Software (SAS Institute, Cary, NC) file for analysis. Data are reported as mean ± 1 s.e.m. PRA is reported both in absolute and natural log terms because values were not normally distributed.
All volunteers who had a baseline and one or more follow-up visits were included in the intention-to-treat analysis. Changes within the RTGT and CHSC groups from baseline to the last study visit were made using the Student's t-test for paired observations. Comparisons between the two groups at baseline, the last study visit, and differences between baseline and the last study visit were performed using the Student's unpaired t-test according to the prespecified analytical plan. The renin values defining low-, medium-, and high-renin patients were prespecified secondary outcomes as was the plan to analyze medication changes and final visit antihypertensive medications according to renin classification. The study was not formally powered to assess the prespecified secondary outcomes, which should be considered exploratory. P values <0.05 were accepted as statistically significant.
Results
The study flow diagram is provided in Figure 1. As shown, 84 patients were randomized, and 5 patients were lost to follow up after the first visit including 2 in RTGT and 3 in CHSC. One patient in each group was withdrawn after the qualifying visit and prior to the first return visit. Seventy-seven patients including 38 in RTGT and 39 in CHSC were included in the intention-to-treat analysis.
The study flow diagram depicts the number of patients who provided informed consent, were randomized to CHSC or RTGT, and were included in the intention-to-treat analysis. BP, blood pressure; CHSC, clinical hypertension specialists' care; ECG, electrocardiogram; ITT analysis, intention-to-treat analysis; RTGT, renin test–guided therapeutics. *Based on clinical judgment.
Baseline characteristics
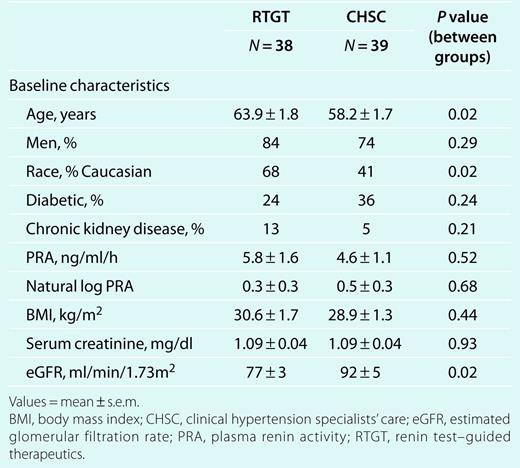
Demographic characteristics of the RTGT and CHSC groups are provided in Table 2. RTGT patients were 6 years older and included a higher proportion of Caucasians. Baseline PRA, expressed as either absolute or natural log values, were not different between the groups despite baseline differences in age, race, and baseline estimated glomerular filtration rate.35
As shown in Table 3, SBP and DBP and baseline antihypertensive medication numbers were not significantly different. Baseline SBP was ≥140 mm Hg in 97% and 90% of the RTGT and CHSC groups, respectively. DBP was ≥90 mm Hg in 50% and 62%, respectively. Final SBPs were comparable, whereas DBP was lower with RTGT (Table 3). The number of antihypertensive medications was similar.
BP and medication number at first and last visits and changes in BP and medication number between the baseline and last visits in RTGT and CHSC patients
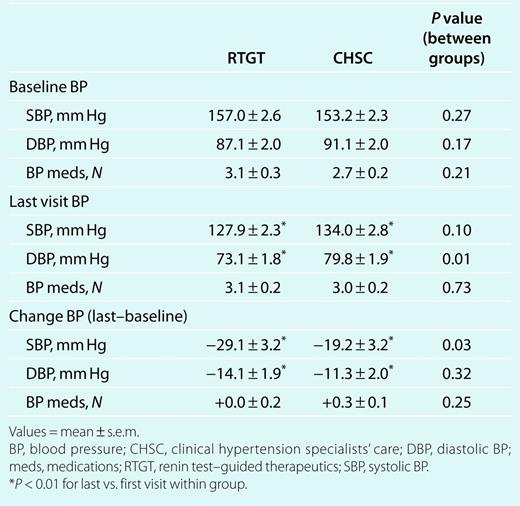 |
 |
BP and medication number at first and last visits and changes in BP and medication number between the baseline and last visits in RTGT and CHSC patients
 |
 |
The mean number of baseline antihypertensive medications was comparable at ~3. In both groups, 22 patients were on ≥3 antihypertensive medications and 7 were on two, whereas 9 patients in RTGT and 10 in CHSC were receiving only one. Nearly all patients receiving two or more antihypertensive medications in both groups were receiving both “R” and “V” agents (29/29 in RTGT; 27/29 in CHSC). The number of antihypertensive medications at the final visit also averaged ~3 in both groups.
Change between first and last study visits
The number of clinic visits was similar with RTGT and CHSC at 3.6 ± 0.1 vs. 3.4 ± 0.2, respectively (range 2–5 in both). DBPs declined significantly in both groups, although the magnitude of the change in DBP between groups was not different (Table 3). SBP fell significantly in both groups. Moreover, SBP declined 10 mm Hg more with RTGT than with CHSC, which was a significant between group difference. The average number of antihypertensive medications did not change in either group.
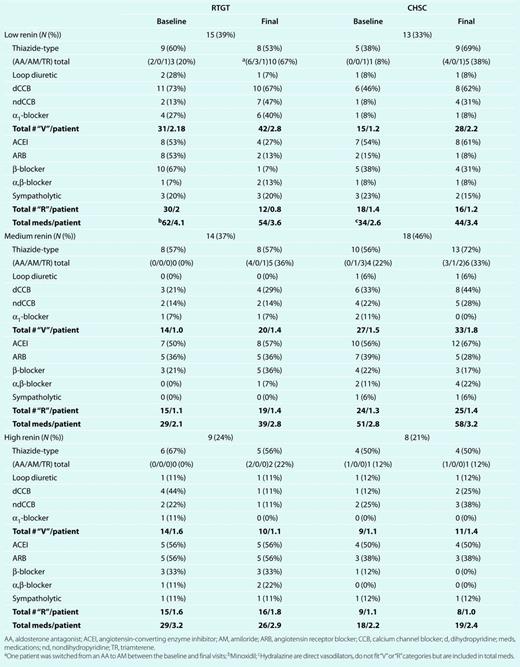
Descriptive changes in “V” and “R” medications between baseline and final visits by renin category are shown in Table 4. CHSC added mostly “V” drugs while making few changes in “R” drugs (Figure 2). In contrast, RTGT subtracted “R” drugs from low-renin patients while adding them to the other groups and subtracted “V” drugs from high-renin patients while adding them to the other groups.
Baseline and final study visit medications in RTGT and CHSC patients by renin subgroup
 |
 |
Baseline and final study visit medications in RTGT and CHSC patients by renin subgroup
 |
 |
The figure illustrates changes in drug usage during the study. There was a 29% reduction in natriuretic antivolume “V” medications in high-renin RTGT patients and a 60% reduction in antirenin “R” antihypertensive drug types in low-renin RTGT patients (left side). In contrast, clinical hypertension specialists' care patients (right side) were mostly treated by adding antivolume “V” medications, although in four patients, the dose of antirenin drugs was increased (not shown here). Low renin (<0.65 ng/ml/h); medium renin (0.65–6.5 ng/ml/h); high renin (>6.5 ng/ml/h). Med, medium; RTGT, renin test–guided therapeutics.
There were also changes in antihypertensive medication doses: Among low-renin RTGT hypertensives, the dose of 4 “V” drugs (CCBs) was up-titrated, while 1 (α1-blocker) was reduced; whereas for CHSC patients, 3 “R” (3 ACEI) and 2 “V” (CCB) medications were up-titrated. For the middle-renin RTGT group, 6 “R” medications (4 ACEI, 1 angiotensin receptor blocker, and 1 β-blocker) and 1 “V” (CCB) were up-titrated; whereas for CHSC patients, 4 “R” (2 ACEI, 1 α,β-blocker, and 1 β-blocker) and 4 “V” (2 CCB, 2 diuretic) medications were up-titrated, while 2 “V” (1 diuretic, 1 CCB) were reduced. Among high-renin patients, the dose was increased in RTGT for 4 “R” medications (2 ACEI, 1 angiotensin receptor blocker, and 1 β-blocker) and in CHSC patients for 4 “R” (2 ACEI and 2 angiotensin receptor blockers) and 2 “V” medications (1 diuretic and 1 CCB).
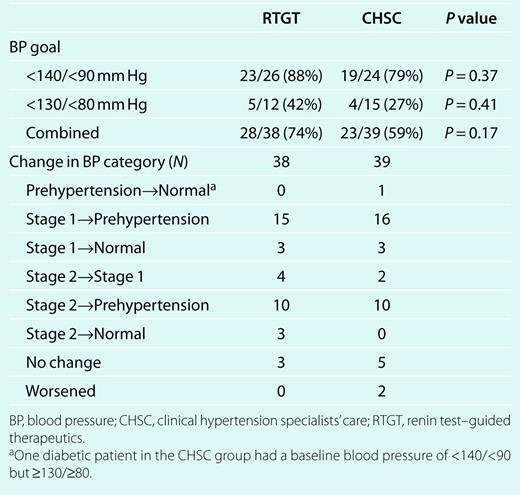
Changes in SBP and DBP for individual patients assigned to RTGT and CHSC are depicted in Figure 3. The percentage of patients achieving the appropriate BP goal (<140/<90 for patients without diabetes and/or chronic kidney disease and <130/<80 mm Hg for patients with one or both of these conditions) is shown in Table 5. Although RTGT patients tended to have higher control rates, the differences vs. CHSC were not statistically significant. This was not a prespecified outcome and was not considered in the power calculations for this study. Similarly, the changes in BP categories shown in Table 5 from the first to last visit in the RTGT and CHSC groups are provided for information only.
Blood pressure control and change in blood pressure category in patients randomized to RTGT and CHSC
 |
 |
Blood pressure control and change in blood pressure category in patients randomized to RTGT and CHSC
 |
 |
The change in systolic (top panel) and diastolic BP (bottom panel) between the first and last study visits is depicted for each patient in the RTGT (left side) and CHSC (right side) groups. Baseline BP readings are indicated by squares and final BP readings are depicted as diamonds. The horizontal lines at 140 systolic and 90 mm Hg diastolic for the patients without diabetes and/or chronic kidney disease and at 130 systolic and 80 mm Hg diastolic for patients with one or both conditions are included to facilitate assessment of BP control. BP, blood pressure; CHSC, clinical hypertension specialists' care; CKD, chronic kidney disease; RTGT, renin test–guided therapeutics.
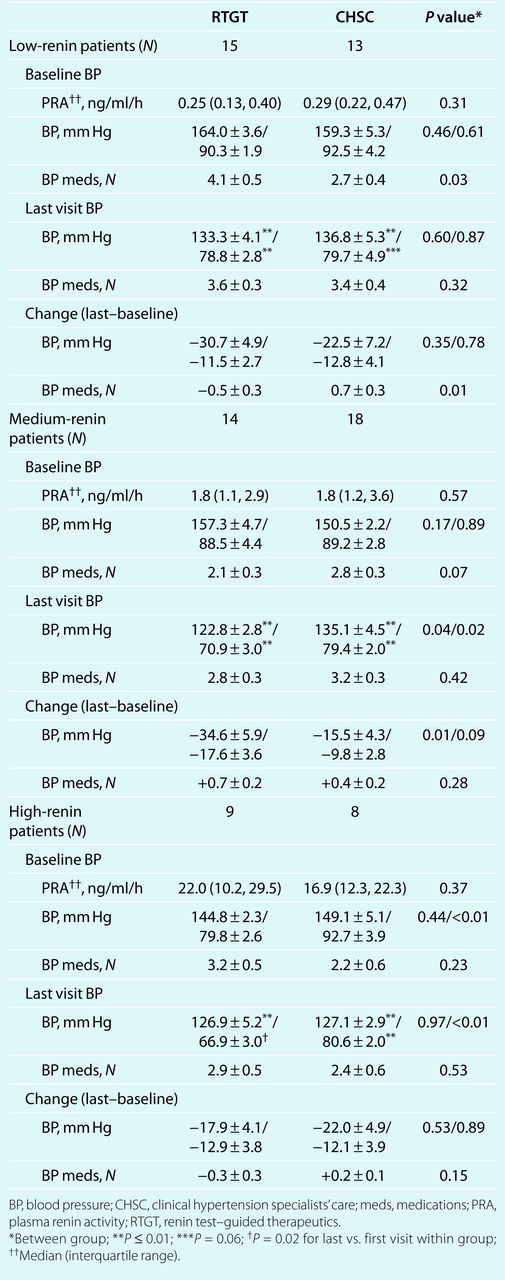
The following subgroup results should be considered exploratory, as this proof-of-concept study was not powered to formally assess these prespecified secondary outcomes. All three renin categories of the RTGT and CHSC groups achieved significant BP reductions (Table 6). Among low-renin patients, baseline antihypertensive medication number was higher in RTGT than CHSC patients and medication number declined with RTGT but increased with CHSC; the net difference was significant. Medium-renin RTGT patients achieved lower treatment SBP and DBP and a significantly greater reduction of BP than CHSC patients. High-renin RTGT patients had lower baseline DBPs and achieved lower treatment DBPs than high-renin CHSC patients. It is of note that the high-renin patients had PRA levels almost 10 times higher than the medium-renin patients and 50–100 times higher than the low-renin patients (Table 6).
Absolute levels and changes in blood pressure and number of medications in plasma renin–based subsets of RTGT and CHSC patients between the baseline and last study visits
 |
 |
Absolute levels and changes in blood pressure and number of medications in plasma renin–based subsets of RTGT and CHSC patients between the baseline and last study visits
 |
 |
Discussion
A recent American Heart Association Scientific Statement defines an urgent need for clinical studies to determine appropriate pharmacologic treatment strategies for resistant hypertension.33 To this end, in the present study, we compared the effectiveness of CHSC, without knowledge of the PRA, to a plasma RTGT algorithm.18 BP was corrected to goal in 59% of CHSC and 74% of RTGT patients. The final BPs were 128 ± 2/73 ± 2 for RTGT and 134 ± 3/80 ± 2 for CHSC. Thus, both treatment groups achieved clinically relevant reductions in BP without any increase in the number of drugs. Moreover, although blood for PRA was not drawn under unusual controlled circumstances and antihypertensive medications were not stopped, patients assigned to the RTGT algorithm achieved a significant advantage in SBP reduction compared to patients in CHSC.
In the RTGT algorithm, PRA testing in treated, uncontrolled hypertensive patients is used to guide further antihypertensive drug selections. Whenever the BP is elevated and PRA is concurrently suppressed, the hypertension is assumed to reflect a body sodium-volume excess. This applies whether PRA is being suppressed by drugs such as β-adrenergic blockers or other sympatholytic agents,25,36 or by excessive sodium retention. Similarly, if BP remains elevated and PRA is not suppressed, plasma renin is assumed to sustain the hypertension.
Although the study was not powered to assess differences within the renin subgroups, the exploratory analyses may be instructive in view of the literature. For example, although study patients averaged three antihypertensive medications at baseline, most of which can increase PRA, 36% presented with low baseline PRAs. In fact, 13 of 15 low-renin RTGT patients were receiving both “R”- and “V”-type antihypertensive medications at baseline. In this low-renin RTGT group, 18 “R”-type medications were withdrawn, whereas only 11 “V”-type medications were added. Similarly, Eide et al.37 found that as many as 67% of their drug-resistant hypertensive patients had low entry PRA. They were also able to correct the hypertension by adding a diuretic with the potassium-sparing and salt-volume depleting drug, amiloride, again verifying the concept that already treated low-renin patients have salt-volume excess “V” hypertension.
Perhaps because the study physicians were unaware of the PRA values, among 13 low-renin CHSC patients, drug numbers actually increased because only 2 “R”-type medications were withdrawn, whereas 13 “V”-type medications were added. Thus, although BPs improved similarly in both low-renin groups (Table 5), RTGT lowered BP just as well as CHSC but with a net reduction in medications.
In this study, CCBs and α-blockers were classified as natriuretic antivolume “V” drugs because they selectively lower BP in low-renin hypertension.22,23 Furthermore, CCBs persistently increase renal sodium excretion,38,39 and hydrochlorothiazide does not enhance this natriuresis.40 CCBs cause sustained natriuresis by intrarenal vasodilation that increases glomerular filtration rate and renal blood flow,38,39 especially to the inner renal medulla, which is critical to sodium-volume homeostasis.41 CCBs also depress sodium reabsorption by binding to α1-receptors in proximal renal tubules.42 α-blockers exert similar but milder intrarenal effects.43 Thus, despite the fact that CCBs can induce pretibial and/or periorbital edema,44 these agents lower BP by inducing sustained natriuresis.
Medium-renin RTGT patients also had greater falls in SBP than medium-renin CHSC patients, but their medications increased from an average of 2.1 to 2.8 with RTGT and from 2.8 to 3.2 with CHSC. (With the full Laragh method18 that forms the basis of the RTGT algorithm, unnecessary “V” or “R” drugs are routinely subtracted after successful BP control. However, this companion algorithm for medication withdrawal18 was not included in the current RTGT protocol).
The hypertension of high-renin patients was successfully treated by both RTGT and CHSC. In RTGT, “R” drug number increased in one patient and was up-titrated in four, whereas four “V” medications were withdrawn. In CHSC, although “R” drug number fell by one, 4 “R” drugs were up-titrated, whereas two “V” medications were added and two were up-titrated (Table 3).
The overall success of the RTGT algorithm demonstrates that using ambulatory PRA testing in treated but uncontrolled hypertensives provides key information about their current sodium-volume status and/or renin status that can be used to improve BP control. It therefore becomes logical to treat low-renin patients primarily with a natriuretic “V” drug and to discontinue “R” drugs in the absence of compelling indications. Similarly, it proved logical to treat “R” hypertensives primarily with an antirenin–angiotensin system “R” medication, while subtracting any “V” drugs, especially if the current PRA is excessively high (above 6.5 ng/ml/h).
Altogether, the success of the renin test–guided treatment algorithm verifies in clinical practice the vasoconstriction-volume analytical model,17 which supports the view that there are two reciprocating long-term supports of BP levels in all of us.45–47 These are (i) the extracellular fluid and plasma volume and/or (ii) long-term angiotensin II–mediated vasoconstriction created by the plasma renin. The results of the present study strongly support this conceptual model.
Study limitations
RTGT patients were older and had a higher proportion of African Americans. Despite these randomization failures, baseline BPs, the average PRA, and the distribution of low-, medium-, and high-renin patients did not differ significantly between the two treatment groups. Although the somewhat higher baseline SBP in the RTGT group may have contributed to their greater decline of SBP, older age and higher SBPs are the strongest predictors of failure to control BP.33 Yet, BP was controlled to goal in 74% of patients in RTGT as compared to 59% in CHSC (P = 0.17).
An alternative strategy for treating uncontrolled hypertension has been to simply add an aldosterone antagonist48,49 or a diuretic/amiloride combination37 to the regimen. In the current trial, BP declined by a mean of 29/14 mm Hg in RTGT patients without any net increase in medication number. The potential benefits of taking less medication include lower cost, fewer adverse effects, and improved adherence, which may counterbalance or exceed the benefits of merely adding an aldosterone antagonist in all patients.
In summary, already treated but uncontrolled hypertension contributes to the substantial public health burden of preventable cardiovascular and renal disease. Our study results indicate that clinical hypertension specialists' referral and renin test–guided therapy are both effective pathways for improving BP control in treated, uncontrolled hypertension. Although referral to a hypertension specialist can improve BP control,15 the number of such specialists is quite insufficient to manage the estimated 17 million treated but uncontrolled hypertensive patients in the US.2,3 Accordingly, the RTGT algorithm emerges as a practical and objective biochemical alternative to CHSC that can be used in most clinical settings by a wide range of health-care providers for addressing the public health burden of treated and uncontrolled hypertension. Reliable commercially available plasma renin assays are becoming widely available, and we have shown here that the test does not require any change in baseline medications or diet or any unusual conditions for blood sample collection and processing. Further studies to document the effectiveness of renin test–guided treatment in a wide variety of clinical settings are in order.
Acknowledgments
This research was supported by grants from the New York Presbyterian Hospital, National Institutes of Health HL04290, and the Ralph H. Johnson Veterans Affairs Hospital.
Disclosure
Based on activities during the previous 5 years, the authors have the following relationships to disclose. B.M.E.; Speaker: AstraZeneca, Boehringer-Ingelheim, Daiichi-Sankyo, Forrest, GlaxoSmithKline, Merck, Novartis, Pfizer; Consultant: AstraZeneca, Forrest, Novartis, Pfizer; Royalties: Hanley & Belfus; Grant support: Abt/Daiichi-Sankyo, Agency for Healthcare Research and Quality, American Society of Hypertension, AstraZeneca, CV Therapeutics, Merck, National Heart, Lung, and Blood Institute, National Center for Minority Health and Health Disparities, New York Presbyterian Hospital, Novartis, Pfizer, RTI International, South Carolina Department of Health and Environmental Control, State of South Carolina, and US Department of Health and Human Services. J.N.B.; Speaker: Abbott, Boehringer-Ingelheim, Daiichi-Sankyo, Forest, GlaxoSmithKline, Merck, Novartis, Pfizer; Consultant: Abbott, Boehringer Ingelheim, Daiichi-Sankyo, Forest, Novartis; Grant support: National Heart, Lung, and Blood Institute, Boehringer-Ingelheim, Novartis. S.U.R.; None. P.B.D.; None. C.H.G.; None. J.F.R.; None. C.A.W.; None. D.T.L., DrPH; None. C.M.; None. J.E.S., DSc; spouse of John Laragh; Consultant: DiaSorin Inc., Stillwater, MN, USA; Renin Laboratory, Temple University School of Medicine; Stock holdings: Quest Diagnostics Inc., Laboratory Corporation of America (both sold out in 2008). John H. Laragh, MD; US Patents #6,595,926 B1 (2003), and #6,632,180 B1 (2003). Method for evaluating and treating hypertension by using renin testing to determine whether to use an antisodium-volume V or an antirenin R drug type for each patient. Patents Licensed: DiaSorin Inc., Stillwater, MN, USA. Consultant: DiaSorin Inc., Stillwater, MN, USA.
References
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.
36.
37.
38.
39.
40.
41.
42.
43.
44.
45.
46.
47.
48.
49.