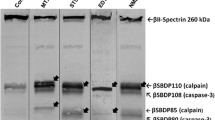
Abstract
Maitotoxin is a potent toxin that activates voltage and receptor-mediated Ca2+ channels, resulting in Ca2+ overload and rapid cell death. We report that maitotoxin-induced cell death is associated with activation of calpain but not caspase-3 proteases in septo-hippocampal cell cultures. Calpain and caspase-3 activation were examined by accumulation of protease-specific breakdown products to α-spectrin. Cell death manifested exclusively necrotic-like characteristics including round, shrunken nuclei, even distribution of chromatin, absence of DNA fragmentation and failure of protein synthesis inhibition to reduce cell death. Necrotic cell death was observed in neurons and astroglia. Calpain inhibitor II inhibited calpain-specific processing of α-spectrin and significantly reduced cell death. The pan-caspase inhibitor, Z-D-DCB, nominally attenuated cell death. Results suggest that: (1) calpain, but not caspase-3, is activated as a result of maitotoxin-induced Ca2+ influx; (2) necrotic cell death caused by maitotoxin exposure is partially mediated by calpain activation; (3) maitotoxin is a useful tool to investigate pathological mechanisms of necrosis.
Similar content being viewed by others
REFERENCES
Melloni, E., and Pontremoli, S. 1989. The calpains. TINS 12:438–444.
Mellgren, R.L., Mericle, M.T., and Lane, R.D. 1986. Proteolysis of the calcium-dependent protease inhibitor by myocardial calcium-dependent protease. Arch. Biochem. Biophys. 246(1):233–9.
Saido, T.C., Sorimachi, H., and Suzuki, K. 1994. Calpain: new perspectives in molecular diversity and physiological-pathological involvement. FASEB J. 8:814–822.
Suzuki, K., Sorimachi, H., Yoshizawa, T., Kinbara, K., and Ishiura, S. 1995. Calpain: novel family members, activation and physiological function. Biol. Chem. 376:523–529.
Sorimachi, H., Harada, K., Saido, T.C., Ono, T., Kawashima, S-I., and Yoshida, K-I. 1997. Downregulation of calpastatin in rat heart after brief ischemia and reperfusion. J. Biochem. 122:743–748.
Hamakubo, T., Kannagi, R., Murachi, T., Matus, A. 1986. Distribution of calpains I and II in rat brain. J. Neurosci. 6(11):3103–11.
Perlmutter, L.S., Gall, C., Baudry, M., and Lynch, G. 1990. Distribution of calcium-activated protease calpain in the rat brain. J. Comp. Neurol. 296:269–276.
Li, Z., Hogan, E.L., and Banik, N.L. 1996. Role of calpain in spinal cord injury: increased calpain immunoreactivity in rat spinal cord after impact trauma. Neurochemical Res. 21(4):441–8.
Wang, K.K.W., Villalobo, A., and Roufogalis, B.D. 1989. Calmodulin-binding proteins as calpain substrates. Biochem. J. 262:693–706.
Kampfl, A., Posmantur, R.M., Zhao, X., Schmutzhard, E., Clifton, G.L., and Hayes, R.L. 1997. Mechanisms of calpain proteolysis following traumatic brain injury: implications for pathology and therapy; implications for pathology and therapy: a review and update. J. Neurotrauma 14:121–134.
Yuen, P., Gilbertsen, R.B., and Wang, K.K.W. 1996. Non-erythroid α-spectrin breakdown by calpain and interleukin 1β-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem. J. 319:683–690.
Bartus, R.T. 1997. The calpain hypothesis of neurodegeneration: Evidence for a common cytotoxic pathway. Neuroscientist 3:314–327.
Nath, R., McGinnis, K.J., Nadimpalli, R., Stafford, D., and Wang, K.K.W. 1996. Effects of ICE-like proteases and calpain inhibitors on neuronal apoptosis. Neuroreport 8:249–255.
Nath, R., Raser, K.J., Stafford, D., Hajimohammadreza, I., Posner, A., Allen, H., Talanian, R.V., Nitatori, T., Sato, N., Waguri, S., Karasawa, Y., Araki, H., Shibanai, K., Kominami, E., and Uchiyama, Y. 1995. Delayed neuronal death in the CA1 pyramidal cell layer of the gerbil hippocampus following transient ischemia is apoptosis. J. Neurosci. 15:1001–1011.
Martin, S.J., O'Brien, G.A., Nishioka, W.K., McGahon, A.J., Mahboubi, A., Saido, T.C., and Green, D.R. 1995. Proteolysis of fodrin (non-erythroid spectrin) during apoptosis. J. Biol. Chem. 270:6425–6428.
Squier, M.K.T., Miller, A.C.K., Malkinson, A.M., and Cohen, J.J. 1994. Calpain activation in apoptosis. J. Cell. Physiol. 159:229–237.
Pike, B.R., Zhao, X., Newcomb, J.K., Wang, K.K.W., Posmantur, R.M., and Hayes, R.L. 1998. Temporal relationships between De Novo protein synthesis, calpain and caspase-3 (CPP32) protease activation, and DNA fragmentation during apoptosis in septo-hippocampal cultures. J. Neurosci. Res., In press.
Behrens, M.M., Marinez, J.L., Moratilla, C., and Renart, J. 1995. Apoptosis induced by protein kinase-C inhibition in a neuroblastoma cell line. Cell Growth Diff. 6:1375–1380.
Jordan, J., Galindo, M.F., and Miller, R.J. 1997. Role of interleukin-1 β converting enzyme-like proteases in the β-amyloid-induced death of rat hippocampal neurons in culture. J. Neurochem. 68:1612–1621.
Eldadah, B.A., Yakovlev, A.G., and Faden, A.I. 1997. The role of CED-3-related cysteine proteases in apoptosis of cerebellar granule. J. Neurosci. 17(16):6105–6113.
Fraser, A., and Evan, G. 1996. A license to kill. Cell 85(6):781–4.
Miura, M., Zhu, H., Rotello, R., Hartwieg, E.A., and Yuan, J. 1993. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell 75(4):653–60.
Zhivotovsky, B., Burgess, D.H., Vanags, D.M., and Orrenius, S. 1997. Involvement of cellular proteolytic machinery in apoptosis. Biochem. Biophys. Res. Comm. 230:481–488.
Cohen, G.M. 1997. Caspases: the executioners of apoptosis. Biochem. J. 326:1–16.
Wang, K.K.W., Posmantur, R.M., M., Nath, R., McGinnis, K., Whitton, M., Talanian, R.V., Glartz, S.B., and Morrow, J.S. 1998. Simultaneous degradation of α II and β-II spectrin by caspase 3 (CPP32) in apoptotic cells. J. Biol. Chem., In press.
Nath, R., Raser, K.J., Stafford, D., Hajimohammadreza, I., Posner, A., Allen, H., Talanian, R.V., Yuen, P., Gilbertsen, R.B., and Wang, K.K. 1996. Non-erythroid alpha-spectrin breakdown by calpain and interleukin 1 beta-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochemical J. 319 (Pt 3):683–90.
Nath, R., Robert, A., Mcginnis, K.M., and Wang, K.K.W. 1998. Evidence for activation of caspase-3-like protease in excitotoxins and hipoxia/hypoglycemia-injured cerebrocortical neurons. J. Neurochem., In press.
Falcieri, E., Marrtelli, A.M., Bareggi, R., Cataldi, A., and Cocco, L. 1993. The protein kinase inhibitor staurosporine induces morphological changes typical of apoptosis in MOLT-4 cells without concomitant DNA fragmentation. Biochem. Biophys. Res. Comm. 193:19–25.
Armstrong, R.C., Aja, T., Xiang, J., Gaur, S., Krebs, J.F., Hoang, K., Bai, X., Korsmeyer, S.J. Karanewsky, D.S., Fritz, L.C., and Tomaselli, K.J. 1996. Fas-induced activation of the cell deathrelated protease CPP32 Is inhibited by Bcl-2 and by ICE family protease inhibitors. J. Biol. Chem. 27(28):16850–5.
Wang, K.K.W., Nath, R., and Raser, K.J. 1996. Hajimohammadreza I. Maitotoxin induces calpain activation in SH-SY5Y neuroblastoma cells and cerebrocortical cultures. Arch. Biochem. Biophys. 331(2):208–14.
Gusovsky, F., and Daly, J.W. 1990. Maitotoxin: a unique pharmacological tool for research on calcium-dependent mechanisms. Biochem. Pharmacol. 39(11):1633–9.
Soergel, D.G., Yasumoto, T., Daly, J.W., and Gusovsky, F. 1992. Maitotoxin effects are blocked by SK&F 96365, an inhibitor of receptor-mediated calcium entry. Mol. Pharmacol. 41:487–493.
Meucci, O., Grimaldi, M., Scorziello, A., Govoni, S., Bergamaschi, S., Yasumoto, T., and Schettini, G. 1992. Maitotoxin-induced intracellular calcium rise in PC12 cells: involvement of dihydropyridine-sensitive and omega-conotoxin-sensitive calcium channels and phosphoinositide breakdown. J. Neurochem. 59:679–688.
Musgrave, I.F., Seifert, R., and Schultz, G. 1994. Maitotoxin activates cation channels distinct from the receptor-activated nonselective cation channels of HL-60 cells. Biochem. J. 301:437–441.
Regan, R.F., Panter, S.S., Witz, A., Tilly, J.L., and Giffard, R.G. 1995. Ultrastructure of excitotoxic neuronal death in murine cortical culture. Brain Res. 705(1–2):188–98.
Roberts-Lewis, J.M., Savage, M.J., Marcy, V.R., Pinsker, L.R., and Siman, R. 1994. Immunolocalization of calpain I-mediated spectrin degradation to vulnerable neurons in the ischemic gerbil brain. J. Neurosci. 14(6):3934–44.
Wang, K.K.W. 1998. An overview of calpain. Bio-reguladores, In press.
Kampfl, A., Zhao, X., Whitson, J.S., Posmantur, R., Dixon, C.E., Yang, K., Clifton, G.L., and Hayes, R.L. 1996. Calpain inhibitors protect against depolarization induced neurofilament protein loss of septo-hippocampal neurons in culture. Eur. J. Neurosci. 8:344–352.
Koh, J., Wie, M.B., Gwag, B.J., Sensi, S.L., Canzoniero, M.T., Demaro, J., Csernansky, C., and Choi, D.W. 1995. Staurosporine-induced neuronal apoptosis. Exp. Neurol. 135:153–159.
Martin, D.P., Schmidt, R.E., DiStefano, P.S., Lowry, O.H., Carter, J.G., and Johnson, E.M. 1988. Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J. Cell. Biol. 106:829–844.
Jones, K.H., and Senft, A.J. 1985. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J. Histochem. Cytochem 33:77–79.
Gong, J., Traganos, F., and Darzynkiewicz, Z. 1994. A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Analyt. Biochem. 218(2):314–319.
Terao, K., Ito, E., Sakamaki, Y., Igarashi, K., Yokoyama, A., and Yasumoto, T. 1998. Histopathological studies of experimental marine toxin poisoning. II. The acute effects of maitotoxin on the stomach, heart and lymphoid tissues in mice and rats. Toxicon 26(4):395–402, 1988.
Oberhammer, F., Fritsch, G., Schmied, M., Pavelka, M., Printz, D., Purchio, T., Lassmann, H., and Schulte-Hermann, R. 1993. Condensation of the chromatin at the membrane of an apoptotic nucleus is not associated with activation of an endonuclease. J. Cell. Science 104:317–326.
Gusovsky, F., Yasumoto, T., and Daly, J.W. 1987. Maitotoxin stimulates phosphoinositide breakdown in neuroblastoma hybrid NCB-20 cells. Cell. Mol. Neurobiol. 7(3):317–22.
Berta, P., Sladeczek, F., Derancourt, J., Durand, M., Travo, P., and Haiech, J. 1986. Maitotoxin stimulates the formation of inositol phosphates in rat aortic myocytes. FEBS Lett. 197(1–2):349–52.
Choi, O.H., Padgett, W.L., Nishizawa, Y., Gusovsky, F., Yasumoto, T., and Daly, J.W. 1990. Maitotoxin: effects on calcium channels, phosphoinositide breakdown, and arachidonate release in pheochromocytoma PC12 cells. Mol. Pharmacol. 37(2):222–30.
Mattson, M.P. 1998. Modification of ion homestasis by lipid peroxidation-roles in neuronal degeneration and adaptive plasticity. TINS 21(2):53–57.
Widdowson, P.S., Gyte, A., Upton, R., Foster, J., Coutts, C.T., and Wyatt, I. 1997. Calpain activation and not oxidative damage mediates L-2-chloropropionic acid-induced cerebellar granule cell necrosis. Tox. Appl. Pharmacol. 142(2):248–55.
Newcomb, J.K., Kampfl, A., Posmantur, R.M., Zhao, X., Pike, B.R., Liu, S.J., Clifton, G.L., and Hayes, R.L. 1997. Immunohistochemical study of calpain-mediated breakdown products to alpha-spectrin following controlled cortical impact injury in the rat. J. Neurotrauma 14(6):369–83.
Vanags, D.M., Porn-Ares, M.I., Coppola, S., Burgess, D.H., and Orrenius, S. 1996. Protease involvement in fodrin cleavage and phosphatidylserine exposure in apoptosis. J. Biol. Chem. 271:31075–31081.
Kluck, R.M., Bossy-Wetzel, E., Green, D.R., and Newmeyer, D.D. 1997. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275:1132–1136.
Kutty, R.K., Singh, Y., Santostasi, and G., Krishna, G. 1989. Maitotoxin-induced liver cell death involving loss of cell ATP following influx of calcium. Tox. Appl. Pharmacol. 101(1):1–10.
Santostasi, G., Kutty, R.K., Bartorelli, A.L., Yasumoto, T., and Krishna, G. 1990. Maitotoxin-induced myocardial cell injury: calcium accumulation followed by ATP depletion precedes cell death. Tox. Appl. Pharmacol. 102(1):164–73.
Jenkins, L.W., Moszynski, K., Lyeth B.G., Lewelt, W., DeWitt, D.S., Allen, A., Dixon, C.E., Povlishock, J.T., Majewski, T.J., Clifton, G.L., Young, H.F., Becker, D.P., and Hayes, R.L. 1989. Increased vulnerability of the mildly traumatized brain to cerebral ischemia: the use of controlled secondary ischemia as a research tool to identify common or different mechanisms contributing to mechanical and ischemic brain injury. Brain Injury 477:211–224.
Lyeth, B.G., Jenkins, L.W., Hamm, R.J., Dixon, C.E., Phillips, L.L., Clifton, G.L., Young, H.F., and Hayes, R.L. 1990. Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 526(2):249–58.
Haseldonckx, M., Van Reempts, J., Van de Ven, M., Wouters, L., and Borgers, M. 1997. Protection with lubeluzole against delayed ischemic brain damage in rats. A quantitative histopathologic study. Stroke 28(2):428–32.
Higuchi, T., Graham, S.H., Fernandez, E.J., Rooney, W.D., Gaspary, H.L., Weiner, M.W., and Maudsley, A.A. 1997. Effects of severe global ischemia on N-acetylaspartate and other metabolites in the rat brain. Magnetic Resonance Med. 37(6):851–7.
Kawaguchi, K., Huerbin, M., and Simon, R.P. 1997. Lesioning of deep prepiriform cortex protects against ischemic neuronal necrosis by attenuating extracellular glutamate concentrations. J. Neurochem. 69(1):412–7.
Cortez, S.C., McIntosh, T.K., and Noble, L.J. 1989. Experimental fluid percussion brain injury: vascular disruption and neuronal and glial alterations. Brain Res. 482(2):271–82.
Qian, L., Nagaoka, T., Ohno, K., Tominaga, B., Nariai, T., Hirakawa, K., Kuroiwa, T., Takakuda, K., and Miyairi, H. 1996. Magnetic resonance imaging and pathologic studies on lateral fluid percussion injury as a model of focal brain injury in rats. Bulletin of Tokyo Medical & Dental University 43(3):53–66.
Colicos, M.A., and Dash, P.K. 1996. Apoptotic morphology of dentate gyrus granule cells following experimental cortical impact injury in rats: possible role in spatial memory deficits. Brain Res. 739:120–131.
Fan, L., Young, P.R., Barone, F.C., Feuerstein, G.Z., Smith, D.H., and McIntosh, T.K. 1996. Experimental brain injury induces differential expression of tumor necrosis factor-alpha mRNA in the CNS. Mol. Brain Res. 36(2):287–91.
Honkaniemi, J., Massa, S.M., Breckinridge, M., and Sharp, F.R. 1996. Global ischemia induces apoptosis-associated genes in hippocampus. Mol. Brain Res. 42:79–88.
MacManus, J.P., Buchan, A.M., Hill, I.E., Rasquinha, I., and Preston, E. 1993. Global ischemia can cause DNA fragmentation indicative of apoptosis in rat brain. Neurosci. Lett. 164:89–92.
Nitatori, T., Sato, N., Waguri, S., Karasawa, Y., Araki, H., Shibanai, K., Kominami, E., and Uchiyama, Y. 1995. Delayed neuronal death in the CA1 pyramidal cell layer of the gerbil hippocampus following transient ischemia is apoptosis. J. Neurosci. 15(2):1001–11.
Conti, A.C., Raghupathi, R., Becher, O., Rink, A.D., Trojanowski, J.O., and McIntosh, T.K. 1996. Delayed apoptotic cell death following lateral fluid percussion brain injury in the rat: a long-term study. J. Neurotrauma 13(10):595 [abstract].
Rink, A., Fung, K., Trojanowski, J.Q., Lee, V.M., Neugebauer, E., and McIntosh, T.K. 1995. Evidence of apoptotic cell death after experimental traumatic brain injury in the rat. Am. J. Pathol. 147:1575–1583.
Yakovlev, A.G., Knoblach, S.M., Fan, L., Fox, G.B., Goodnight, R., and Faden, A.I. 1997. Activation of CPP32-like caspases contributes to neuronal apoptosis and neurological dysfunction after traumatic brain injury. J. Neurosci. 17(19):7415–7424.
Rosser, B.G., Powers, S.P., and Gores, G.J. 1993. Calpain activity increases in hepatocytes following addition of ATP. Demonstration by a novel fluorescent approach. J. Biol. Chem. 268(31):23593–600.
Takahashi, M., Ohizumi, Y., and Yasumoto, T. 1982. Maitotoxin, a Ca2+ channel activator candiate. J. Biol. Chem. 257(13):7287–89.
Gottron, F.J., Ying, H.S., and Choi, D.W. 1997. Caspase inhibition selectively reduces the apoptotic component of oxygen-glucose deprivation-induced cortical neuronal cell death. Mol. Cell. Neurosci. 9(3):159–69.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhao, X., Pike, B., Newcomb, J. et al. Maitotoxin Induces Calpain But Not Caspase-3 Activation and Necrotic Cell Death in Primary Septo-Hippocampal Cultures. Neurochem Res 24, 371–382 (1999). https://doi.org/10.1023/A:1020933616351
Issue Date:
DOI: https://doi.org/10.1023/A:1020933616351