Abstract
Information on the prognosis of patients with transient ischaemic attack or moderately disabling ischaemic stroke associated with bilateral internal carotid artery (ICA) occlusion is scarce. We prospectively studied 57 consecutive patients (46 men; mean age 60 ± 9 years) with bilateral ICA occlusion who had presented with unilateral transient or moderately disabling cerebral or retinal ischaemic symptoms. We determined the long-term risk of recurrent ischaemic stroke and the composite outcome of stroke, myocardial infarction or vascular death. Four patients had a recurrent ischaemic stroke during a mean follow-up of 5.9 years, resulting in an annual stroke rate of 1.2% (95% confidence interval (CI) 0.3–3.1). Risk factors for recurrent ischaemic stroke could not be identified. Eighteen patients suffered a stroke, myocardial infarction or vascular death, resulting in an annual rate for major vascular events of 5.3% (95% CI 3.1–8.3). Age and a history of ischaemic heart disease were significant risk factors for future vascular events. Patients with transient or moderately disabling symptoms of cerebral or retinal ischaemia associated with bilateral ICA occlusion have a relatively low risk of recurrent ischaemic stroke. Although this study was not designed to compare conservative treatment with surgical intervention, the favourable outcome suggests that a policy of medical therapy and control of risk factors may be justified in these patients.
Similar content being viewed by others
Introduction
A transient ischaemic attack (TIA) or ischaemic stroke is associated with complete occlusion of one of the internal carotid arteries (ICA) in about 9% of patients in hospital based series [13, 20]. In a single study of 2,228 patients with TIA or stroke only eight patients (0.4%) had bilateral ICA occlusion [20]. Information on the management and prognosis of patients with bilateral occlusion of the ICA is limited by the small numbers of patients studied and the short duration of follow-up in the majority of studies [1, 4, 8, 9, 11, 18, 21, 25–27]. Some studies on outcome of patients with TIA or stroke associated with bilateral ICA occlusion have emphasized a high risk of recurrent ischaemic stroke [1, 8, 11] and therefore have recommended surgical revascularization by means of an extracranial–intracranial (EC/IC) bypass or endarterectomy of an external carotid artery (ECA). In contrast, a meta-analysis of the outcome of patients with transient or moderately disabling signs and symptoms associated with ICA occlusion suggested that patients with a bilateral ICA occlusion may fare as well or even better than patients with unilateral ICA occlusion [16]. When confronted with a patient with symptomatic bilateral ICA occlusion in clinical practice, information on the risk of new vascular events is essential to weigh the risks and benefits of treatment other than antithrombotic medication and control of vascular risk factors, such as revascularization procedures.
We performed a longitudinal study of a large series of consecutive patients with bilateral ICA occlusion who had presented with transient or moderately disabling cerebral or retinal ischaemia to determine the long-term risk of recurrent ischaemic stroke and other major vascular outcome events and to describe haemodynamic characteristics in relation to the risk of recurrent stroke.
Patients and methods
Patients
We prospectively included consecutive patients who were referred to the Department of Neurology of the University Medical Center of Utrecht, The Netherlands, between January 1990 and February 2007 because of bilateral ICA occlusion that had caused transient (lasting <24 h) or at most moderately disabling symptoms of ischaemia of the eye or brain (modified Rankin score ≤3 [3]). Transient ischaemic attack (TIA) or stroke was defined as the acute onset of neurological deficit, without signs of haemorrhage on computed tomography (CT) scan or magnetic resonance imaging (MRI). Ischaemic symptoms of the eye included transient monocular blindness, retinal infarction or chronic ocular ischaemia [5]. The bilateral ICA occlusion (100% obstruction, defined as absence of contrast filling of both ICAs) was preferentially documented by conventional digital subtraction angiography. If magnetic resonance angiography or duplex ultrasonography already had demonstrated the absence of flow in both ICAs, conventional angiography was performed only in patients in whom visualisation of the collateral circulation was important in considering a surgical revascularization procedure. We excluded patients with dissection or ICA occlusion associated with radiation-induced vasculopathy. All patients were interviewed at baseline about their symptoms and about vascular risk factors as listed in Table 1. This included specific questions about symptoms suggestive of a haemodynamic origin of TIAs or stroke, e.g. limb-shaking, retinal claudication or occurrence of TIAs or stroke subsequent to rising from a sitting or lying position or to exercise, extensive blood loss, cardiac failure, postprandial hypotension or transition from a cold to warm environment [17]. We also documented whether patients continued to suffer from neurological symptoms after bilateral ICA occlusion had been diagnosed. The study was approved by the medical ethics committee of the University Medical Center Utrecht.
Collateral pathways and transcranial Doppler CO2-reactivity
The presence of stenosis in the subclavian arteries, common carotid arteries (CCAs), ECAs, vertebral arteries (VAs), basilar artery, and collateral blood flow pathways were assessed on the digital subtraction angiograms. The degree of stenosis was measured according to the NASCET criteria [10] and dichotomised as a stenosis of ≥50% or less than that. Collateral blood flow pathways were studied for the symptomatic hemisphere. Collateral blood flow via the ophthalmic artery (OphthA) was considered present if selective catheterisation of the CCA showed filling of intracranial arteries distal from the carotid syphon via the ECA and OphthA. Collateral blood flow via the posterior communicating artery (PComA) was considered present if selective catheterisation of one of the VAs showed filling of the anterior cerebral artery (ACA) or middle cerebral artery (MCA) branches via the PComA. Angiography of the posterior circulation was also used to study leptomeningeal collateral blood supply from the posterior cerebral artery (PCA) to the vascular territory of the ACA and MCA.
All patients underwent transcranial Doppler (TCD) with assessment of the CO2-reactivity to investigate cerebrovascular reserve capacity [17]. The CO2-reactivity after carbogene inhalation was measured as the relative change in blood flow velocity in the MCA and expressed as a percentage. A CO2-reactivity of <20% was considered abnormal, since this value corresponds with the mean CO2-reactivity minus two times the standard deviation (SD) in normal controls [14].
Treatment and follow-up
All patients received antithrombotic medication and management of vascular risk factors. Long-term monitoring of medication compliance and risk factor control was left to the general practitioner. Patients with ≥70% stenosis of the ECA on the symptomatic side were offered carotid endarterectomy (CEA). Patients with frequently recurring cerebral symptoms and haemodynamic features as described above or low TCD CO2-reactivity were offered EC/IC bypass operation according to the method of Tulleken [15, 23, 24]. Follow-up was performed by telephone interviews of patients, their relatives or their general practitioners. All possible events of recurrent ischaemic or haemorrhagic stroke were verified by revision of the medical records and CT scan or MRI of the brain. The primary outcome event was fatal or non-fatal ischaemic stroke, defined as a new neurological deficit that persisted for more than 24 h, and that was not associated with signs of haemorrhage on CT scan or MRI of the brain. The secondary outcome event was the composite event of fatal or non-fatal ischaemic or haemorrhagic stroke, myocardial infarction or vascular death, whichever occurred first. Causes of vascular death included terminal heart failure, sudden death, systemic bleeding, pulmonary embolism or complications after vascular surgery.
Data analysis
The annual risks for the primary and secondary outcome event were calculated, with 95% confidence intervals (CI). The baseline characteristics as listed in Table 1 were compared between patients with and without subsequent ischaemic stroke. The association of vascular risk factors with the outcome events was assessed by a Cox proportional hazards model and expressed as hazard ratio (HR) with 95% CI. Risk factors that showed an association with the outcome event with a p-value < 0.15 in the univariable analysis were included in a multivariable model.
Results
A total of 57 patients (mean age 60, range 42–79 years; 46 men) were included. Thirty-six (63%) patients had presented with moderately disabling ischaemic stroke, 12 (21%) with cerebral TIA and nine (16%) patients with retinal ischaemic symptoms only (Table 1). Retinal ischaemic symptoms included transient monocular blindness in five, retinal infarction in two and chronic ocular ischaemic syndrome in two patients. Of the 48 patients with cerebral ischaemic symptoms, four patients had had retinal ischaemic symptoms as well. In 36 (63%) of 57 patients the left hemisphere or eye had been symptomatic and in 21 (37%) the right hemisphere or eye. Ten (18%) patients had had clinical symptoms suggestive of a haemodynamic origin, such as limb-shaking or precipitation of symptoms after rising or exercise. In 23 (40%) of 57 patients neurological symptoms had continued after the bilateral carotid occlusion had been documented. TCD CO2-reactivity on the symptomatic side was below 20% in 28 of 42 (67%) patients and on the asymptomatic side in 25 of 43 (58%) patients. None of the patients had a stenosis ≥70% of the ECA on the symptomatic side. Two patients underwent an uncomplicated EC/IC bypass operation and were censored at the time of operation.
Collateral blood flow patterns
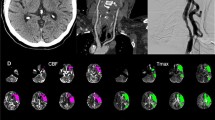
Cerebral angiography was performed in 43 (75%) patients. The presence of additional lesions in cerebropetal arteries and collateral blood flow patterns are shown in Table 2. The majority of patients had collateral flow via the posterior circulation (90%). Twenty-three (70%) patients had collateral blood flow towards the symptomatic hemisphere via the OphthA, but in none of these patients this was the only collateral blood flow pathway present. Three patients showed no collateral flow via the PComA; in one of these a connection was visualised between the vertebral artery and the distal ICA with filling of ACA and MCA branches. In the two other patients the perfusion of the symptomatic hemisphere was dependent on flow via the OphthA and leptomeningeal vessels. The angiogram of three patients showed collateral flow via the OphthA on the asymptomatic side through the anterior communicating artery (AComA), with filling of the ACA in the symptomatic hemisphere in one patient and of the ACA and MCA in the symptomatic hemisphere in two patients (Fig. 1).
Angiogram of a 64-year-old man with bilateral ICA occlusion with collateral blood flow towards the left symptomatic hemisphere via the OphthA on the asymptomatic side, who did not suffer a recurrent ischaemic stroke during a follow-up period of 3.2 years; a bilateral ICA occlusion; b selective catheterisation of the left CCA shows filling of only a few MCA branches via the OphthA; c selective catheterisation of the right CCA shows extensive filling of ACA and MCA branches in the right hemisphere and d of the left hemisphere via the right OphthA and subsequently the AComA with filling of ACA and MCA branches
Follow-up: primary outcome measure
Mean duration of follow-up was 5.9 years (range 2 months–16.5 years). Of the 57 patients, four (7%) men had a recurrent ischaemic stroke, which was fatal in 2 patients. The corresponding annual stroke rate was 1.2% (95% CI 0.3–3.1). Table 1 summarises the baseline characteristics of patients with and without a recurrent ischaemic stroke. Of the four patients with recurrent stroke, none had presented with only retinal ischaemic symptoms. One of the four patients had presented with symptoms suggestive of a haemodynamic origin. Two of the patients with recurrent stroke had had ongoing neurological symptoms after the bilateral ICA occlusion had been documented. TCD CO2-reactivity on the symptomatic or asymptomatic side did not differ between patients with and without recurrent stroke. Table 3 shows the clinical characteristics and the type of collateral pathways utilised at baseline of the four patients with recurrent ischaemic stroke. Because of the small number of recurrent ischaemic strokes, Cox proportional hazards modelling of possible determinants of recurrent ischaemic stroke, including the type of collateral pathways and TCD CO2-reactivity could not be performed.
Follow-up: secondary outcome measure
Eighteen (32%) patients had an ischaemic or haemorrhagic stroke, myocardial infarction or died from a vascular cause, corresponding to an annual event rate of 5.3% (95% CI 3.1–8.3). In addition to four ischaemic strokes, four patients suffered from an intracerebral haemorrhage (fatal in three) and four patients had non-fatal myocardial infarction. Other vascular causes of death apart from stroke were cardiac failure in two patients, sudden death in three patients and complications after peripheral vascular surgery in one patient. The intracerebral haemorrhage was located in the basal ganglia with intraventricular extension in three patients and in the fourth patient the diagnosis was based on the symptoms of acute headache, vomiting and a hemiparesis followed by death. Age (HR 1.07, 95% CI 1.01–1.14) and a history of ischaemic heart disease (HR 3.6, 95% CI 1.3–10) were associated with the secondary outcome measure (Table 4). In a multivariable analysis including age, hypertension and a history of ischaemic heart disease, the HR for the composite endpoint associated with age was 1.06 (95% CI 0.99–1.12) and for a history of ischaemic heart disease 2.4 (95% CI 0.8–6.8).
Discussion
This largest longitudinal study to date of patients with bilateral carotid occlusion presenting with transient or moderately disabling symptoms of cerebral or retinal ischaemia shows a relatively low risk of recurrent ischaemic stroke. We could not identify determinants of the risk of recurrent stroke. Age and a history of ischaemic heart disease are risk factors for major vascular events in general (any stroke, myocardial infarction or vascular death).
The previously reported annual stroke rates in patients with bilateral ICA occlusion range between 0 and 13% [1, 4, 8, 9, 11, 18, 21, 25, 26]. The largest prospective follow-up study on bilateral ICA occlusion with non- or moderately disabling symptoms so far was published more than 20 years ago and consisted of 34 patients, of whom 11 patients suffered a stroke during a mean duration of follow-up of 3.5 years [26]. That the annual stroke rate in our study was comparatively low may be partly attributed to the relatively large proportion of patients with retinal ischaemic symptoms included in our study; it has been found before that patients with unilateral occlusion and only symptoms of retinal ischaemia have a lower risk of recurrent stroke than those with cerebral ischaemic symptoms [12, 17]. Another explanation may be that medical treatment for secondary prevention of stroke and control of vascular risk factors is currently more effective than two or three decades earlier. Most other follow-up studies found annual stroke rates between 0 and 6%, which is more in agreement with our findings. However, these included only a small number of patients (between 8 and 21) [1, 18, 21, 25] or described only patients who underwent an EC/IC bypass operation [8] or various revascularization operations including EC/IC bypass [11].
It has been suggested that in patients with bilateral ICA occlusion who survive without major stroke, the collateral blood flow pathways are particularly efficient and maintain cerebral perfusion [25]. We found extensive collateral blood flow patterns in the symptomatic hemisphere in the majority of patients, including the patients who later on had a recurrent stroke. Although the low event rate did not allow a formal analysis of collateral pathways as determinants of recurrent stroke, we found that subsequent infarction occurred not only in patients with additional collateral blood flow via the OphthA or leptomeningeal blood vessels, which are considered secondary pathways [19] but also in patients with collateral blood flow via the PComA without collateral blood flow via secondary pathways. In addition, not all patients with only secondary collateral pathways suffered a recurrent ischaemic stroke. In line with our results, two previous studies of patients with bilateral ICA occlusion also failed to find an association between the type of collateral blood flow pathways and recurrent stroke [26, 27]. We hypothesise that to survive bilateral ICA occlusion without major stroke, extensive collateral blood flow pathways must already have developed at the time of first presentation. This view may be supported by the results of a subgroup analysis of the Asymptomatic Carotid Atherosclerosis Study (ACAS), in which medically treated asymptomatic patients with a stenosis of the ICA of 60% or more and a contralateral ICA occlusion had a lower risk of death (during first 30 days) and stroke (cumulative 5-year rate 3.5%) than patients without contralateral ICA occlusion (cumulative 5-year rate 11.7%) [2].
Since the presence of bilateral ICA occlusion is a sign of very severe atherosclerotic disease, a high rate of other vascular events can be expected. In agreement with other studies, [18, 25] we found that indeed recurrent ischaemic stroke made up only a modest proportion of the vascular events that occurred during the time of follow-up. In previous follow-up studies the risk of stroke, myocardial infarction or vascular death in patients who were not selected because of carotid disease, amounted to 4% per year in patients who were followed for a mean of 10 years after TIA [6] and a cumulative 5-year risk of 29% after first ischaemic stroke [7]. Another study of 2,739 patients with TIA or moderately disabling stroke treated with aspirin or the combination of aspirin and dipyridamole during a mean follow-up time of 3.5 years found an annual risk of stroke, myocardial infarction or vascular death between 3% and 4% [22]. Against this background, symptomatic bilateral occlusion in our series is associated with a comparable event rate of 5.3%.
This study has some limitations. First, the study cohort consisted of patients from all over the Netherlands, who were referred to a single tertiary university hospital and therefore referral bias most probably plays a role. However, patients with frequent and ongoing symptoms will probably have been more readily referred than those with only one event and no further symptoms. In that case, the risk of recurrent stroke that we report would rather be overestimated than underestimated. Second, follow-up was performed by telephone interviews instead of regular hospital visits and events that had only a minor impact on the patient’s abilities may have been missed. Third, all patients were advised about the control of vascular risk factors, but we did not obtain information on how well vascular risk factors were indeed controlled in individual patients. However, despite possible non-compliance with medication we found a relatively low risk of recurrent ischaemic stroke. Fourth, two patients were censored at the time of an EC/IC bypass operation that was advised because of ongoing TIAs. It is uncertain whether these patients might have had an ischaemic stroke if they had not been operated.
In conclusion, this study shows that patients with transient or moderately disabling cerebral or retinal ischaemic symptoms associated with bilateral ICA occlusion have a relatively low risk of recurrent ischaemic stroke and a risk of any major vascular event that is comparable to this risk in patients with TIA or stroke in general. Although this study was not designed to compare conservative treatment with surgical intervention, our observations suggest that a policy of medical therapy and control of risk factors may be justified in these patients.
References
AbuRahma AF, Copeland SE (1998) Bilateral internal carotid artery occlusion: natural history and surgical alternatives. Cardiovasc Surg 6:579–583
Baker WH, Howard VJ, Howard G, Toole JF (2000) Effect of contralateral occlusion on long-term efficacy of endarterectomy in the asymptomatic carotid atherosclerosis study (ACAS). ACAS Investigators. Stroke 31:2330–2334
Banks JL, Marotta CA (2007) Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke 38:1091–1096
Bogousslavsky J, Regli F (1985) Cerebro-retinal ischemia after bilateral occlusion of internal carotid artery. A study with prospective follow-up. Neuroradiology 27:238–247
Carter JE (1985) Chronic ocular ischemia and carotid vascular disease. Stroke 16:721–728
Clark TG, Murphy MF, Rothwell PM (2003) Long term risks of stroke, myocardial infarction, and vascular death in “low risk” patients with a non-recent transient ischaemic attack. J Neurol Neurosurg Psychiatry 74:577–580
Dhamoon MS, Tai W, Boden-Albala B, Rundek T, Paik MC, Sacco RL, Elkind MS (2007) Risk of myocardial infarction or vascular death after first ischemic stroke: the Northern Manhattan Study. Stroke 38:1752–1758
El-Fiki M, Chater NL, Weinstein PR (1985) Results of extracranial–intracranial arterial bypass for bilateral carotid occlusion. J Neurosurg 63:521–525
Fields WS, Lemak NA (1976) Joint study of extracranial arterial occlusion. X. Internal carotid artery occlusion. JAMA 235:2734–2738
Fox AJ (1993) How to measure carotid stenosis. Radiology 186:316–318
Friedman SG, Lamparello PJ, Riles TS, Imparato AM, Sakwa MP (1987) Surgical management of the patient with bilateral internal carotid artery occlusion. J Vasc Surg 5:715–718
Grubb RL Jr, Derdeyn CP, Fritsch SM, Carpenter DA, Yundt KD, Videen TO, Spitznagel EL, Powers WJ (1998) Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 280:1055–1060
Hurwitz BJ, Heyman A, Wilkinson WE, Haynes CS, Utley CM (1985) Comparison of amaurosis fugax and transient cerebral ischemia: a prospective clinical and arteriographic study. Ann Neurol 18:698–704
Klijn CJM, Kappelle LJ, van der Grond J, Visser GH, Algra A, Tulleken CAF, van Gijn J (2001) Lack of evidence for a poor haemodynamic or metabolic state of the brain in patients with haemodynamic clinical features associated with carotid artery occlusion. Cerebrovasc Dis 12:99–107
Klijn CJM, Kappelle LJ, van der Zwan, van Gijn J, Tulleken CAF (2002) Excimer laser-assisted high-flow extracranial/intracranial bypass in patients with symptomatic carotid artery occlusion at high risk of recurrent cerebral ischemia: safety and long-term outcome. Stroke 33:2451–2458
Klijn CJM, Kappelle LJ, Algra A, van Gijn J (2001) Outcome in patients with symptomatic occlusion of the internal carotid artery or intracranial arterial lesions: a meta-analysis of the role of baseline characteristics and type of antithrombotic treatment. Cerebrovasc Dis 12:228–234
Klijn CJM, Kappelle LJ, van Huffelen AC, Visser GH, Algra A, Tulleken CAF, van Gijn J (2000) Recurrent ischemia in symptomatic carotid occlusion: prognostic value of hemodynamic factors. Neurology 55:1806–1812
Lazarides M, Kalodiki E, Williams M, Christopoulos D, Nicolaides AN (1991) Natural history of chronic bilateral internal carotid artery occlusion. Int Angiol 10:209–212
Liebeskind DS (2003) Collateral circulation. Stroke 34:2279–2284
Mead GE, Wardlaw JM, Lewis SC, Dennis MS (2006) No evidence that severity of stroke in internal carotid occlusion is related to collateral arteries. J Neurol Neurosurg Psychiatry 77:729–733
Nicholls SC, Kohler TR, Bergelin RO, Primozich JF, Lawrence RL, Strandness DE Jr (1986) Carotid artery occlusion: natural history. J Vasc Surg 4:479–485
The Esprit study group (2006) Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet 367:1665–1673
Tulleken CAF, Verdaasdonk RM (1995) First clinical experience with excimer assisted high flow bypass surgery of the brain. Acta Neurochir (Wien) 134:66–70
Tulleken CAF, Verdaasdonk RM, Beck RJ, Mali WP (1996) The modified excimer laser-assisted high-flow bypass operation. Surg Neurol 46:424–429
Verhaeghe R, Naert J, Vermylen J (1991) Bilateral carotid artery occlusion: clinical presentation and outcome. Clin Neurol Neurosurg 93:123–126
Wade JP, Wong W, Barnett HJM, Vandervoort P (1987) Bilateral occlusion of the internal carotid arteries. Presenting symptoms in 74 patients and a prospective study of 34 medically treated patients. Brain 110:667–682
Wortzman G, Barnett HJM, Lougheed WM (1968) Bilateral internal carotid occlusion: a clinical and radiological study. Can Med Assoc J 99:1186–1196
Acknowledgments
S. Persoon is supported by a grant from the Netherlands Heart Association (grant number 2003B263). C. J. M. Klijn is supported by a clinical fellowship from the Netherlands Organisation for Health Research and Development (grant number 907-00-103). We thank Professor J. van Gijn, MD, FRCP, FRCPE for his valuable comments on an earlier version of the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Persoon, S., Klijn, C.J.M., Algra, A. et al. Bilateral carotid artery occlusion with transient or moderately disabling ischaemic stroke: clinical features and long-term outcome. J Neurol 256, 1728–1735 (2009). https://doi.org/10.1007/s00415-009-5194-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-009-5194-3