Abstract
Objectives
Subarachnoid hemorrhage (SAH) is a common cause of chronic hydrocephalus. Blood in the subarachnoid space is intracranially metabolized to bilirubin and iron, and free iron is thereafter detoxified by ferritin. However, no studies have reported the relationship between intracranial heme metabolism and chronic hydrocephalus after SAH. The goal of this prospective study was to clarify the relationship between intracranial heme metabolism and chronic hydrocephalus after SAH.
Methods
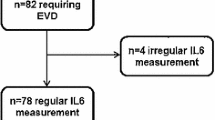
The authors measured the levels of bilirubin, iron and ferritin in the cerebrospinal fluid (CSF) of 70 consecutive patients with aneurysmal SAH of Fisher computed tomography Group III, and determined the relationship between these substances’ levels and hydrocephalus requiring ventriculoperitoneal shunting.
Results
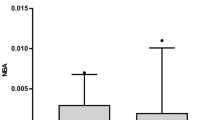
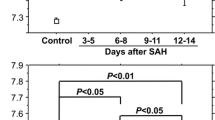
The CSF concentrations of ferritin and inflammatory cells were significantly higher in shunted patients (n = 27) than in non-shunted patients (n = 43) on Days 3 and 4 (p<0.05 in ferritin and p<0.01 in inflammatory cells) and 11 to 14 (p<0.005 in ferritin) post-SAH. These results were independent of other clinical factors. The occurrence of chronic hydrocephalus was not affected by the extent of the intracranial heme metabolism in terms of the bilirubin and iron levels.
Conclusions
This is the first study to show that patients who subsequently had chronic hydrocephalus requiring CSF shunting were associated with higher CSF levels of ferritin in the acute stage of SAH. Higher CSF ferritin levels may not reflect the amount of blood in the subarachnoid space that was intracranially metabolized, but rather more intense subarachnoid inflammatory reactions which may cause chronic hydrocephalus after SAH.



Similar content being viewed by others
References
Alksne JF, Lovings E (1972) The role of the arachnoid villi in the removal of red blood cells from the subarachnoid space: an electron microscopic study in the dog. J Neurosurg 36:192–200
Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM (1992) Ferritin: a cytoprotective antioxidant stratagem of endothelium. J Biol Chem 267:18148–18153
Bradbury MWB (1997) Transport of iron in the blood-brain-cerebrospinal fluid system. J Neurochem 69:443–454
Davalos A, Castillo J, Marrugat J, Fernandez-Real JM, Armengou A, Cacabelos P, Rama R (2000) Body iron stores and early neurologic deterioration in acute cerebral infarction. Neurology 54:1568–1574
Dorai Z, Hynan LS, Kopitnik TA, Samson D (2003) Factors related to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery 52:763–771
Drake CG (1988) Report of World Federation of Neurological Surgeons Committee on a universal subarachnoid hemorrhage grading scale [letter]. J Neurosurg 68:985–986
Everse J, Hsia N (1997) The toxicities of native and modified hemoglobins. Free Radic Biol Med 22:1075–1099
Fisher CM, Kistler JP, Davis JM (1980) Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 6:1–9
Gruber A, Reinprecht A, Bavinzski G, Czech T, Richling B (1999) Chronic shunt-dependent hydrocephalus after early surgical and early endovascular treatment of ruptured intracranial aneurysms. Neurosurgery 44:503–512
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36
Hulet SW, Heyliger SO, Powers S, Connor JR (2000) Oligodendrocyte progenitor cells internalize ferritin via clathrin-dependent receptor mediated endocytosis. J Neurosci Res 61:52–60
Jackowski A, Crockard A, Burnstock G, Russell RR, Kristek F (1990) The time course of intracranial pathophysiological changes following experimental subarachnoid haemorrhage in the rat. J Cereb Blood Flow Metab 10:835–849
Jennett B, Bond M (1975) Assessment of outcome after severe brain damage. Lancet 1:480–484
Kibler RF, Couch RSC, Crompton MR (1961) Hydrocephalus in the adult following spontaneous subarachnoid hemorrhage. Brain 84:45–61
Kim YO, Kang JS, Youm MH, Woo YJ (2003) Diagnostic capability of CSF ferritin in children with meningitis. Pediatr Neurol 28:271–276
Kreiter KT, Copeland D, Bernardini GL, Bates JE, Peery S, Claassen J, Du YE, Stern Y, Connolly ES, Mayer SA (2002) Predictors of cognitive dysfunction after subarachnoid hemorrhage. Stroke 33:200–209
LeVine SM, Lynch SG, Ou CN, Wulser MJ, Tam E, Boo N (1999) Ferritin, transferrin and iron concentrations in the cerebrospinal fluid of multiple sclerosis patients. Brain Res 821:511–515
Massicotte EM, Del Bigio MR (1999) Human arachnoid villi response to subarachnoid hemorrhage: possible relationship to chronic hydrocephalus. J Neurosurg 91:80–84
Ono S, Zhang ZD, Marton LS, Yamini B, Windmeyer E, Johns L, Kowalczuk A, Lin G, Macdonald RL (2000) Heme oxygenase-1 and ferritin are increased in cerebral arteries after subarachnoid hemorrhage in monkeys. J Cereb Blood Flow Metab 20:1066–1076
Ryter SW, Tyrrell RM (2000) The heme synthesis and degradation pathways: role in oxidant sensitivity: heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med 28:289–309
Sajanti J, Heikkinen E, Majamaa K (2000) Transient increase in procollagen propeptides in the CSF after subarachnoid hemorrhage. Neurology 55:359–363
Schmieder K, Koch R, Lucke S, Harders A (1999) Factors influencing shunt dependency after aneurysmal subarachnoid hemorrhage. Zentralbl Neurochir 60:133–140
Sheehan JP, Polin RS, Sheehan JM, Baskaya MK, Kassell NF (1999), Participants Factors associated with hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery 45:1120–1128
Suzuki H, Kanamaru K, Tsunoda H, Inada H, Kuroki M, Sun H, Waga S, Tanaka T (1999) Heme oxygenase-1 gene induction as an intrinsic regulation against delayed cerebral vasospasm in rats. J Clin Invest 104:59–66
Suzuki H, Muramatsu M, Kojima T, Taki W (2003) Intracranial heme metabolism and cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke 34:2796–2800
Synek V, Reuben JR, Du Boulay GH (1976) Comparing Evans’ index and computerized axial tomography in assessing relationship of ventricular size to brain size. Neurology 26:231–233
Takahashi S, Oki J, Miyamoto A, Moriyama T, Asano A, Inyaku F, Okuno A (1999) Beta-2-microglobulin and ferritin in cerebrospinal fluid for evaluation of patients with meningitis of different etiologies. Brain Dev 21:192–199
Takizawa T, Tada T, Kitazawa K, Tanaka Y, Hongo K, Kameko M, Uemura K (2001) Inflammatory cytokine cascade released by leukocytes in cerebrospinal fluid after subarachnoid hemorrhage. Neurol Res 23:724–730
Turner CP, Bergeron M, Matz P, Zegna A, Noble LJ, Panter SS, Sharp FR (1998) Heme oxygenase-1 is induced in glia throughout brain by subarachnoid hemoglobin. J Cereb Blood Flow Metab 18:257–273
Widenka DC, Wolf S, Schurer L, Plev DV, Lumenta CB (2000) Factors leading to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurol Neurochir Pol 34(6 Suppl):56–60
Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G (2003) Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke 34:2964–2969
Zappone E, Bellotti V, Cazzola M, Ceroni M, Meloni F, Pedrazzoli P, Perfetti V (1986) Cerebrospinal fluid ferritin in human disease. Haematologica 71:103–107
Acknowledgements and Funding
None to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Received in revised form: 19 January 2006
Rights and permissions
About this article
Cite this article
Suzuki, H., Muramatsu, M., Tanaka, K. et al. Cerebrospinal fluid ferritin in chronic hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurol 253, 1170–1176 (2006). https://doi.org/10.1007/s00415-006-0184-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-006-0184-1