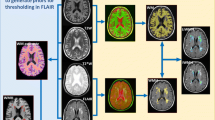
Abstract
Objective
White matter hyperintensities (WMH) are common on brain MRI of the elderly. Their size ranges from punctate to early confluent to confluent lesions. While this increase in extension is frequently seen as evidence for a continuum of changes, histological data and clinical follow-up suggest differences in underlying pathology and their progression.
Methods
We tested this hypothesis by exploring the distributions of punctuate and confluent lesions using lesion probability maps (LPM) generated from MRI scans of 189 participants (mean age 60.8+/−6.2 years) in the Austrian Stroke Prevention Study. We dichotomised WMH according to the classification by Fazekas et al. [punctate (n=143) vs. early confluent and confluent (n=33)] to run voxel-based t-tests using permutation-based nonparametric inference. To test alternative hypotheses, we created similar LPM for age and arterial hypertension.
Results
We observed significant differences in the spatial distribution of lesions for the two WMH groups (p<0.01). Punctate lesions were more diffusely distributed throughout the cerebral white matter (peak probability ∼5%) relative to confluent lesions (peak probability 45%). Confluent lesions had greatest likelihood of being found in perfusion “watershed” regions. These differences in distribution could not be explained by differences in age or hypertension only, as both greater age and the diagnosis of hypertension were associated with WMH abutting the occipital horns.
Conclusions
Punctate and early confluent to confluent WMH show distinguishable differences in their spatial distribution within a normal elderly population. The pattern of punctate WMH is probably a consequence of mixed etiologies. Preferential localization of the more confluent WMH with arterial watershed areas implies a stronger ischemic component in their development.

Similar content being viewed by others
References
de Leeuw FE, de Groot JC, Achten E, et al. (2001) Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 70:9–14
Fazekas F, Kleinert R, Offenbacher H, et al. (1993) Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 43:1683–1689
Schmidt R, Enzinger C, Ropele S, Schmidt H, Fazekas F, the Austrian Stroke Prevention Study (2003) Progression of cerebral white matter lesions: 6-year results of the Austrian Stroke Prevention Study. Lancet 14:2046–2048
Pantoni L (2002) Pathophysiology of Age-Related Cerebral White Matter Changes. Cerebrovasc Dis 13:7–10
De Groot JC, De Leeuw FE, Oudkerk M, et al. (2002) Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol 52:335–341
Schmidt R, Scheltens P, Erkinjuntti T, et al. (2004) White matter lesion progression: A surrogate endpoint for trials in cerebral small-vessel disease. Neurology 63:139–144
Awad IA, Johnson PC, Spetzler RF, Hodak JA (1986) Incidental subcortical lesions identified on magnetic resonance imaging in the elderly II. Postmortem pathological correlations. Stroke 17:1090–1097
Scheltens P, Barkhof F, Leys D, Wolters EC, Ravid R, Kamphorst W (1995) Histopathologic correlates of white matter changes on MRI in Alzheimer’s disease and normal aging. Neurology 45:883–888
Narayanan S, Fu L, Pioro E, et al. (1997) Imaging of axonal damage in multiple sclerosis: spatial distribution of magnetic resonance imaging lesions. Ann Neurol 41:385–391
Lee MA, Smith S, Palace J, et al. (1999) Spatial mapping of T2 and gadolinium-enhancing T1 lesion volumes in multiple sclerosis: evidence for distinct mechanisms of lesion genesis? Brain 122:1261–1270
Charil A, Zijdenbos AP, Taylor J, et al. (2003) Statistical mapping analysis of lesion location and neurological disability in multiple sclerosis: application to 452 patient data sets. NeuroImage 19:532–544
Wen W, Sachdev P (2004) The topography of white matter hyperintensities on brain MRI in healthy 60- to 64-year-old individuals. NeuroImage 22:144–154
DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust W (2005) Anatomical mapping of white matter hyperintensities (WMH). Exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke 36:50–55
Fazekas F, Niederkorn K, Schmidt R, et al. (1988) White matter signal abnormalities in normal individuals: correlation with carotid ultrasonography, cerebral blood flow measurements, and cerebrovascular risk factors Stroke 19:1285–1288
de Leeuw F-E, de Groot JC, Oudkerk M, et al. (2002) Hypertension and cerebral white matter lesions in a prospective cohort study. Brain 125:765–772
Strassburger TL, Hing-Chung L, Daly EM, et al. (1997) Interactive effects of age and hypertension on volumes of brain structures. Stroke 28:1410–1417
Carmelli D, Swan GE, Reed T, Wolf PA, Miller BL, DeCarli C (1999) Midlife cardiovascular risk factors and brain morphology in identical older male twins. Neurology 52:1114–1115
Schmidt R, Fazekas F, Kapeller P, Schmidt H, Hartung HP (1999) MRI white matter hyperintensities: three-year follow-up of the Austrian Stroke Prevention Study. Neurology 53:132–139
1999 World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee. J Hypertens 17:151–183
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am J Roentgenol 49:351–356
Barkhof F, Scheltens P (2002) Imaging of White Matter Lesions. Cerebrovasc Dis 13:21–30
Plummer D (1992) DispImage: a display and analysis tool for medical images. Rev Neuroradiol 5:489–495
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage 17:825–841
Nichols TE, Holmes AP (2002) Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15:1–25
Worsley KJ, Marrett S, Neelin P, Evans AC (1996) Searching scale space for activation in PET images. Hum Brain Mapping 4:74–90
Smith SM, Jenkinson M, Woolrich MW, et al. (2004) Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23:208–219
Longstreth Jr WT, Arnold AM, Beauchamp NJ, et al. (2005) Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly. The Cardiovascular Health Study. Stroke 36:56–61
Acknowledgments and Funding
This work has been supported by the FWF Austrian Science Fund (Vienna; CE and SR, grant number J2373-B02 and P15158, respectively), the UK Medical Research Council (PMM) and an EPSRC Advanced Research Fellowship (SS). We also thank all study participants for their enthusiasm with this project. There are no possible conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Received in revised form: 31 October 2005
Rights and permissions
About this article
Cite this article
Enzinger, C., Smith, S., Fazekas, F. et al. Lesion probability maps of white matter hyperintensities in elderly individuals. J Neurol 253, 1064–1070 (2006). https://doi.org/10.1007/s00415-006-0164-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-006-0164-5