Abstract
Objectives
To investigate the ability of susceptibility-weighted imaging (SWI) to predict stroke evolution in comparison with perfusion-weighted imaging (PWI).
Methods
In a retrospective analysis of 15 patients with non-lacunar ischaemic stroke studied no later than 24 h after symptom onset, we used the Alberta Stroke Program Early CT Score (ASPECTS) to compare lesions on initial diffusion-weighted images (DWI), SWI, PWI and follow-up studies obtained at least 5 days after symptom onset. The National Institutes of Health Stroke Scale scores at entry and stroke risk factors were documented. The clinical–DWI, SWI–DWI and PWI–DWI mismatches were calculated.
Results
SWI–DWI and mean transit time (MTT)–DWI mismatches were significantly associated with higher incidence of infarct growth (P = 0.007 and 0.028) and had similar ability to predict stroke evolution (P = 1.0). ASPECTS values on initial DWI, SWI and PWI were significantly correlated with those on follow-up studies (P ≤ 0.026) but not associated with infarct growth. The SWI ASPECTS values were best correlated with MTT ones (ρ = 0.8, P < 0.001).
Conclusions
SWI is an alternative to PWI to assess penumbra and predict stroke evolution. Further prospective studies are needed to evaluate the role of SWI in guiding thrombolytic therapy.
Key Points
• SWI can provide perfusion information comparable to MTT
• SWI–DWI mismatch can indicate ischaemic penumbra
• SWI–DWI mismatch can be a predictor for stroke evolution
Similar content being viewed by others
Introduction
Contemporary therapy for acute ischaemic stroke is based on the concept of penumbra, which is an area with reduced blood flow but not to such a level that causes irreversible cell membrane failure [1]. Although challenged by several limitations, mismatch between larger abnormal areas on MR perfusion-weighted imaging (PWI) and smaller restricted areas on diffusion-weighted imaging (DWI) is a widely accepted approach to detecting penumbra, predicting stroke evolution and determining patients with the greatest potential to benefit from thrombolytic therapy [2–4]. However, PWI requires administration of a contrast agent, which is contraindicated in a variety of clinical conditions, such as renal insufficiency and previous reactions to contrast agents.
Susceptibility-weighted imaging (SWI) is a high-resolution, three-dimensional, gradient-echo T2* MR technique with enhanced sensitivity for paramagnetic substances, such as blood products, iron and calcifications [5, 6]. SWI has been used in evaluating trauma, vascular malformations, tumours, as well as cerebrovascular and neurodegenerative diseases [7, 8]. In acute ischaemic stroke, severe reduction of cerebral perfusion pressure causes increased deoxyhaemoglobin to oxyhaemoglobin ratio by increasing oxygen extraction fraction (OEF) [9, 10]. Although there is not yet a direct comparison between SWI and positron emission tomography (PET) images, the gold standard for quantifying the OEF, several studies have shown decreased T2* related to increased OEF [11–13]. As a result, SWI can show asymmetric prominent hypointense vessels potentially from different concentrations of deoxyhaemoglobin between ischaemic and normal brain areas. This potential metabolic information on SWI may help to delineate penumbra without contrast agent administration.
We hypothesise that SWI provides comparable perfusion information as PWI does and the SWI–DWI mismatch represents an alternative tool for evaluating ischaemic penumbra in patients with ischaemic stroke.
Materials and methods
Subjects
In an organised registry of our stroke centre, we retrospectively retrieved data on 303 consecutive patients with acute stroke admitted between January 2009 and September 2010. Inclusion criteria were:
-
1.
Complete stroke MR sequences, including DWI, SWI and PWI no later than 24 h after symptom onset
-
2.
Acute non-lacunar infarcts in the territory of the middle cerebral artery identified as hyperintensities on DWI and confirmed as hypointensities on apparent diffusion coefficient (ADC) maps
-
3.
Follow-up MR fluid-attenuated inversion recovery (FLAIR) images or CT obtained at least 5 days after symptom onset for assessment of final infarct (FI) size
Patients with haemorrhagic transformation or poor image quality were excluded from the analysis.
Overall, a total of 19 patients fulfil the inclusion criteria with adequate imaging quality. Among these, 4 patients (21.1%) developed haemorrhagic transformations on follow-up studies. As a result, 15 patients (7 women, 8 men; median age 68 years; range 35–89) met the study criteria. They presented with symptoms of hemiplegia or hemiparesis with or without dysphasia or aphasia. The National Institutes of Health Stroke Scale (NIHSS) scores at entry and discharge were documented. Risk factors for ischaemic stroke, including hypertension, diabetes mellitus and hyperlipidaemia, were obtained by chart review. The study was approved by our institutional review board and conformed to ethical standards.
Imaging techniques
Magnetic resonance imaging studies were performed with a 1.5-T MR system (Magnetom Avanto; Siemens Medical Solutions, Erlangen, Germany) by using a 12-channel head coil. The following sequences were obtained: axial T1 spin echo, T2 fast spin echo and FLAIR sequences. All these sequences were done with a slice thickness of 5 mm, a gap of 1 mm and a matrix of 256 × 206 pixels. The DWI was acquired using an isotropic single-shot echo-planar sequence with the following parameters: repetition time/echo time (TR/TE) 3,700/109, b max 1,000 s/mm2, slice thickness 5 mm, matrix 128 × 128 pixels. The trace images and ADC maps were calculated. For the SWI sequence, the magnitude and phase images were obtained with the following parameters: TR/TE 49/40, flip angle 15°, bandwidth 80 kHz, slice thickness 2 mm with 64 slices in a single slab, iPAT factor of 2 and a matrix of 177 × 256 pixels. Post-processing was performed and 2-mm mIP images were generated. PWI data were acquired using an echo-planar imaging sequence after an intravenous bolus injection of GBCA (Omniscan, GE Healthcare, USA), which was typically delivered by a power injector through a 22-ga intravenous line at a rate of 3 mL/s. The dynamic image acquisition started 35–40 s before the contrast agent entered the brain and ended after a few recirculation passes of contrast agent, with the first pass approximately in the middle of the acquisition. The PWI used the following parameters: TR/TE 2,220/45, slice thickness 5 mm, matrix 128 × 100 pixels. Mean transit time (MTT), relative cerebral blood flow (rCBF) and relative cerebral blood volume (rCBV) maps were created by post-processing of the concentration–time curves for each pixel on a commercially available workstation (DynaSuite Neuro, Invivo, Orlando, FL, USA).
The follow-up CT images were acquired using 64-slice CT equipment (Somatom Sensation 64; Siemens Medical Solutions, Forchheim, Germany) with a slice thickness of 5 mm.
Image analysis
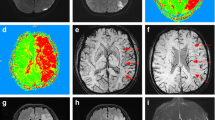
All images were collected from our picture archiving and communication system as DICOM-format data and imported to an Osirix image viewer (Version 3.8.1, 32 bit) on an Apple iMac for analysis. A 10-point semi-quantitative CT scoring system, Alberta Stroke Program Early CT Score (ASPECTS) [14], was used to assess the size abnormal signal intensities or densities on individual DWI, SWI, PWI and FI studies. Two neuroradiologists (H.W.K. and F.Y.T.), blinded to patient information except for the clinical history of ischaemic stroke, independently assessed ASPECTS values on the selected images. For calculating ASPECTS values, 1 point was subtracted from 10 for an area of ischaemic changes, including restricted diffusion, asymmetric prominent vessels on SWI, prolonged MTT, reduced rCBF and rCBV, hyperintensity on FLAIR images and hypointensity on CT (Fig. 1). Image contrast and brightness were optimised between areas of ischaemic changes and normal-appearing brain.
An example of ASPECTS assessment on a 54-year-old woman. Initial MR imaging was performed 7 h after symptom onset. The 10 ASPECTS regions are C caudate, L lentiform nucleus, IC internal capsule, I insular ribbon, M1–6 cortical regions of the middle cerebral artery (M1–3 at the level of basal ganglia, M4–6 at the level rostral to the ganglionic structures). a On diffusion-weighted images, the ASPECTS value is 7 because of hyperintensities in the left I, M2, M5. On b the mean transit time (MTT) map and c susceptibility-weighted images, the ASPECTS values are 3 as areas of prolonged MTT or asymmetric prominent vessels involve I and M1–6. d On follow-up fluid-attenuated inversion recovery images, obtained 47 days after symptom onset, the ASPECTS value is 5 as the hyperintensities involve the I, L, M2, M4 and M5
According to the relationship between ASPECTS values on initial DWI and FI studies, patients were classified as infarct growth (IG) group (DWI > FI), non-infarct growth (NIG) group (DWI = FI), or DWI reversal group (DWI < FI). Mismatches were determined if DWI ASPECTS values were greater than SWI or PWI ones. Patients with clinical–DWI mismatch were documented if both entry NIHSS and DWI ASPECTS value were at least 8, a cut-off point used by Tei et al. [15]. The presence of thrombus on SWI was defined as blooming linear hypointensities along the course of the cerebral arteries. The locations of the thrombi were documented by the raters. Disagreements between the raters were resolved by consensus.
Statistical analysis
Non-parametric tests were used because the data were not normally distributed. The clinical variables, ASPECTS values, and mismatches were given as mean (SD), median (range) or number (percentage). Group comparisons were performed by using Mann–Whitney U tests for ordinal variables and Fisher’s exact tests for categorical variables. To detect differences among ASPECTS values on various imaging parameters, non-parametric repeated measures analysis of variance (Friedman and Wilcoxon signed ranks tests) were used. The correlations between the ASPECTS values on various imaging parameters and NIHSS were measured by using Spearman’s rank correlation tests. Regression lines were computed. A statistical software package (SPSS, version 17.0; SPSS, Chicago, IL, USA) was used for the above analyses. To compare the abilities of SWI–DWI and MTT–DWI in predicting stroke evolution, McNemar’s test (MedCalc, version 11.5.1.0; MedCalc software, Mariakerke, Belgium) was used. Results were considered significant at the 5% level, two-tailed.
Results
In a total of 15 patients, 6 (40%) were classified into the IG group, the others into the NIG group. No patient showed reversal of DWI lesions. The initial MR imaging was performed at a median of 5 h (range 1.5–18) after symptom onset. FI studies were obtained at a median of 7 days (range 5–60) after symptom onset. Ten patients (66.7%) were followed with CT and 5 (33.3%) with MR imaging.
Cerebral ischaemic infarcts were in the territory of the right middle cerebral artery in 7 patients (46.7%) and in the territory of the left one in 8 patients (53.3%). Fourteen patients (93.3%) showed thrombi responsible for the infarcts on SWI. Most of the thrombi (n = 13, 92.9%) were located in the M1 segment of the middle cerebral artery, whereas one (7.1%) was in the M2 segment. One patient in the NIG group was treated with intravenous tissue plasminogen activator without imaging evidence of recanalisation. Three patients in the IG group underwent intra-arterial thrombolytic treatments and only one of them showed successful recanalisation. The rest of the patients (n = 11, 73.3%) were treated conservatively.
The clinical variables and imaging parameters are summarised in Table 1. There were no significant differences in the clinical variables and ASPECTS values on initial imaging parameters between IG and NIG groups. The entry NIHSS and clinical–DWI mismatch rate were higher in the IG group than in the NIG group, but the differences between groups did not reach statistical significance. Among the mismatch parameters, only SWI–DWI and MTT–DWI were significantly associated with IG (P = 0.007 and 0.028). The difference between the abilities of SWI–DWI and MTT–DWI mismatches to predict infarct growth was 6.7% (95% confidence interval −6.3, 6.7; P = 1).
Figure 2 gives an overview of the ASPECTS values on various imaging parameters after grouping patients into IG and NIG groups. A significant difference in ASPECTS values among the parameters was found in the IG group (P < 0.001), but not in the NIG group (P = 0.202). In the IG group, the DWI ASPECTS values were not significantly different from rCBF and rCBV values (P = 0.368). The SWI ASPECTS values were not significantly different from MTT and FI values (P = 0.779), but significantly lower than DWI values (P = 0.028).
Table 2 shows correlations among various imaging parameters. There were significant correlations in the ASPECTS values among all initial imaging parameters and FI studies. The rCBF was the most accurate parameter to predict FI (ρ = 0.875, P < 0.001). The ASPECTS values on SWI correlated significantly with those on other initial imaging parameters, best with the values on MTT (ρ = 0.8, P < 0.001, Fig. 3).
Among the 15 patients, 4 patients died without follow-up NIHSS. For the remaining 11 patients, the discharge NIHSS correlated most significantly with entry NIHSS (ρ = 0.952, P < 0.001, Table 3). The ASPECTS values on DWI, SWI, rCBF, rCBV and FI also significantly correlated with the discharge NIHSS, and best with the values on FI studies (ρ = −0.761, P = 0.006).
Discussion
The present study is the first to incorporate SWI for predicting stroke evolution. With the use of the ASPECTS system, individual DWI, SWI and PWI were all positively correlated with FI studies, but not associated with IG. The SWI was best correlated with MTT and the SWI–DWI and MTT–DWI mismatches had a similar ability to forecast IG.
The standard method of measuring infarct size on images is manual volumetry. However, volumetry may lead to inaccurate estimation of infarct size because of early oedematous change or late shrinkage [16, 17]. Furthermore, clinicians often prefer qualitative visual estimation of the mismatch rather than time-consuming volumetry. The ASPECTS system is a reliable grading system that assesses the extent of ischaemic change and predicts functional outcome in patients with acute ischaemic stroke [14, 18, 19]. The application of ASPECTS has been extended to MR stroke imaging and found to be an ideal rapid screening tool for calculating PWI–DWI mismatch with good inter-rater agreement [20, 21]. We took the advantage of this practical grading system to test our hypothesis that SWI is an alternative to PWI for detecting penumbra and predicting stroke evolution.
Although the clinical applicability of the mismatch concept is still controversial, PWI–DWI mismatch is widely used to assess penumbra and shown to be associated with a higher risk of IG in a number of studies [22, 23]. Our results revealed that SWI–DWI and MTT–DWI mismatches had a similar capability to predict stroke evolution. In addition, the close relationship between the ASPECTS values of SWI and MTT implied an association between cerebral OEF and MTT. In a PET study by Yata et al. [24], there was a significant elevation of OEF related to increased MTT. Kamath et al. [25] also found that MTT was the best parameter correlated with OEF in a correlation study between perfusion CT and PET. With the potential metabolic information similar to MTT and OEF, SWI can be an ideal alternative to help define penumbra without contrast agent administration, which is hazardous in patients with compromised renal function. Furthermore, SWI has the advantage of a high degree of sensitivity in detecting clot formation and cerebral microbleeds, information which is vital in managing stroke patients [26, 27].
Although SWI–DWI and MTT–DWI mismatches were associated with IG in our study, accurate prediction of IG remains a challenge in individual patients. Ischaemic stroke is heterogeneous and factors affecting stroke evolution are multiple. Our data showed that patients with SWI–DWI or MTT–DWI mismatches did not always progress to having IG. In addition, it is known that IG may occur in the setting of matched PWI and DWI. In terms of stroke evolution, lesions on DWI are generally thought to represent infarct core and do not reduce in size. However, DWI lesions have been shown to reverse in a small number of patients [16, 28]. In our study, the reversibility of DWI was not present.
For prediction of FI size in acute ischaemic stroke, there is no consensus regarding which MR imaging parameter is most accurate [22, 29–31]. In a study by Karonen et al. [32], lesions on MR perfusion parameters (MTT, rCBF, rCBV) all correlated with FI volumes, findings consistent with those of our current study. The best correlation between rCBF and FI size (ρ = 0.875, P < 0.001) found in our study is in line with a study by Parsons et al. (ρ = 0.91, P < 0.001) [3]. Furthermore, we demonstrated a positive correlation between the ASPECTS values of SWI and FI. However, these correlations between initial and FI studies only indicated conceivable FI sizes proportional to their initial counterparts. None of the individual imaging parameters was predictive of IG.
Our study has several limitations. First, given the limited number of patients in this study, the statistical power was not optimal. The associations between ASPECTS values on various imaging parameters with P < 0.05 should be considered marginally significant, whereas the association between SWI and MTT with P < 0.01 showed a stronger correlation. Second, many patients in our stroke registry were excluded from the analysis mainly because of haemorrhage or incomplete imaging studies. In our institution, SWI has been a routine study for stroke for years, whereas PWI is usually performed to evaluate candidates for intra-arterial thrombolytic therapy. Third, the follow-up imaging investigations were not uniform. However, with incorporation of anatomical information, the ASPECTS system has the advantage of diminishing both potential inter-investigation scoring differences and a comparison problem resulting from oedema or shrinkage of infarcted areas. On the other hand, the ASPECTS system is bound to be insensitive to subtle IG that occurs inside the initial allotted territories. Finally, some of our patients undertook thrombolytic therapy, whereas others did not. However, this heterogeneity may not confound the test of the hypothesis because the potential benefit of the treatments appeared trivial in our study.
In conclusion, our results show that SWI provides comparable information to MTT. SWI–DWI mismatch can be a marker for ischaemic penumbra and a predictor of stroke evolution. Further prospective studies of SWI are needed to investigate its role in guiding thrombolytic therapy.
References
Astrup J, Siesjo BK, Symon L (1981) Thresholds in cerebral ischemia – the ischemic penumbra. Stroke 12:723–725
Barber PA, Darby DG, Desmond PM et al (1998) Prediction of stroke outcome with echoplanar perfusion- and diffusion-weighted MRI. Neurology 51:418–426
Parsons MW, Yang Q, Barber PA et al (2001) Perfusion magnetic resonance imaging maps in hyperacute stroke: relative cerebral blood flow most accurately identifies tissue destined to infarct. Stroke 32:1581–1587
Davis SM, Donnan GA, Butcher KS et al (2005) Selection of thrombolytic therapy beyond 3 h using magnetic resonance imaging. Curr Opin Neurol 18:47–52
Haacke EM, Xu Y, Cheng YC et al (2004) Susceptibility weighted imaging (SWI). Magn Reson Med 52:612–618
Reichenbach JR, Venkatesan R, Schillinger DJ et al (1997) Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology 204:272–277
Sehgal V, Delproposto Z, Haacke EM et al (2005) Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging 22:439–450
Tsui YK, Tsai FY, Hasso AN et al (2009) Susceptibility-weighted imaging for differential diagnosis of cerebral vascular pathology: a pictorial review. J Neurol Sci 287:7–16
Derdeyn CP, Yundt KD, Videen TO et al (1998) Increased oxygen extraction fraction is associated with prior ischemic events in patients with carotid occlusion. Stroke 29:754–758
Grubb RL Jr, Derdeyn CP, Fritsch SM et al (1998) Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 280:1055–1060
Lee JM, Vo KD, An H et al (2003) Magnetic resonance cerebral metabolic rate of oxygen utilization in hyperacute stroke patients. Ann Neurol 53:227–232
Li D, Wang Y, Waight DJ (1998) Blood oxygen saturation assessment in vivo using T2* estimation. Magn Reson Med 39:685–690
An H, Lin W (2000) Quantitative measurements of cerebral blood oxygen saturation using magnetic resonance imaging. J Cereb Blood Flow Metab 20:1225–1236
Barber PA, Demchuk AM, Zhang J et al (2000) Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet 355:1670–1674
Tei H, Uchiyama S, Usui T (2007) Clinical-diffusion mismatch defined by NIHSS and ASPECTS in non-lacunar anterior circulation infarction. J Neurol 254:340–346
Chemmanam T, Campbell BC, Christensen S et al (2010) Ischemic diffusion lesion reversal is uncommon and rarely alters perfusion-diffusion mismatch. Neurology 75:1040–1047
Harrer JU (2010) Clinical applicability and the perfusion-diffusion mismatch theory: not yet a perfect match. Neurology 75:1034–1035
Hill MD, Rowley HA, Adler F et al (2003) Selection of acute ischemic stroke patients for intra-arterial thrombolysis with pro-urokinase by using ASPECTS. Stroke 34:1925–1931
Pexman JH, Barber PA, Hill MD et al (2001) Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol 22:1534–1542
Butcher K, Parsons M, Allport L et al (2008) Rapid assessment of perfusion-diffusion mismatch. Stroke 39:75–81
Tei H, Uchiyama S, Usui T et al (2011) Diffusion-weighted ASPECTS as an independent marker for predicting functional outcome. J Neurol 258:559–565
Schaefer PW, Hunter GJ, He J et al (2002) Predicting cerebral ischemic infarct volume with diffusion and perfusion MR imaging. AJNR Am J Neuroradiol 23:1785–1794
Kane I, Sandercock P, Wardlaw J (2007) Magnetic resonance perfusion diffusion mismatch and thrombolysis in acute ischaemic stroke: a systematic review of the evidence to date. J Neurol Neurosurg Psychiatry 78:485–491
Yata K, Suzuki A, Hatazawa J et al (2006) Relationship between cerebral circulatory reserve and oxygen extraction fraction in patients with major cerebral artery occlusive disease: a positron emission tomography study. Stroke 37:534–536
Kamath A, Smith WS, Powers WJ et al (2008) Perfusion CT compared to H2 (15)O/O (15)O PET in patients with chronic cervical carotid artery occlusion. Neuroradiology 50:745–751
Nandigam RN, Viswanathan A, Delgado P et al (2009) MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR Am J Neuroradiol 30:338–343
Mittal S, Wu Z, Neelavalli J et al (2009) Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol 30:232–252
Olivot JM, Mlynash M, Thijs VN et al (2009) Relationships between cerebral perfusion and reversibility of acute diffusion lesions in DEFUSE: insights from RADAR. Stroke 40:1692–1697
Karonen JO, Vanninen RL, Liu Y et al (1999) Combined diffusion and perfusion MRI with correlation to single-photon emission CT in acute ischemic stroke. Ischemic penumbra predicts infarct growth. Stroke 30:1583–1590
Perkio J, Soinne L, Ostergaard L et al (2005) Abnormal intravoxel cerebral blood flow heterogeneity in human ischemic stroke determined by dynamic susceptibility contrast magnetic resonance imaging. Stroke 36:44–49
Kane I, Carpenter T, Chappell F et al (2007) Comparison of 10 different magnetic resonance perfusion imaging processing methods in acute ischemic stroke: effect on lesion size, proportion of patients with diffusion/perfusion mismatch, clinical scores, and radiologic outcomes. Stroke 38:3158–3164
Karonen JO, Liu Y, Vanninen RL et al (2000) Combined perfusion- and diffusion-weighted MR imaging in acute ischemic stroke during the 1st week: a longitudinal study. Radiology 217:886–894
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kao, HW., Tsai, F.Y. & Hasso, A.N. Predicting stroke evolution: comparison of susceptibility-weighted MR imaging with MR perfusion. Eur Radiol 22, 1397–1403 (2012). https://doi.org/10.1007/s00330-012-2387-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2387-4