Abstract
Purpose
Some of the neuroprotective effects of hydrogen sulfide (H2S) have been attributed to systemic hypometabolism and hypothermia. However, systemic metabolism may vary more dramatically than brain metabolism after cardiac arrest (CA). The authors investigated the effects of inhaled exogenous hydrogen sulfide on brain metabolism and neurological function in rabbits after CA and resuscitation.
Methods
Anesthetized rabbits were randomized into a sham group, a sham/H2S group, a CA group, and a CA/H2S group. Exogenous 80 ppm H2S was administered to the sham/H2S group and the CA/H2S group which suffered 3 min of untreated CA by asphyxia and resuscitation. Effects on brain metabolism (cerebral extraction of oxygen (CEO2), arterio-jugular venous difference of glucose [AJVD(glu)] and lactate clearance), S100B, viable neuron counts, neurological dysfunction score, and survival rate were evaluated.
Results
CEO2, AJVD(glu), and lactate increased significantly after CA. Inhalation of 80 ppm H2S significantly increased CEO2 (25.04 ± 7.11 vs. 16.72 ± 6.12 %) and decreased AJVD(glu) (0.77 ± 0.29 vs. 1.18 ± 0.38 mmol/L) and lactate (5.11 ± 0.43 vs. 6.01 ± 0.64 mmol/L) at 30 min after resuscitation when compared with the CA group (all P < 0.05). In addition, neurologic deficit scores, viable neuron counts, and survival rate were significantly better whereas S100B was decreased after H2S inhalation.
Conclusions
The present study reveals that inhalation of 80 ppm H2S reduced neurohistopathological damage and improves early neurological function after CA and resuscitation in rabbits. The increased CEO2 and decreased AJVD(glu) and enhanced lactate clearance may be involved in the protective effects.
Similar content being viewed by others
Introduction
Over the last decade, hydrogen sulfide (H2S) has been recognized as a gasotransmitter, playing an important role in brain function [1–3]. Endogenous H2S can be produced from cysteine by cystathionine β-synthase (CBS) in the central nervous system and cystathionine γ-lyase (CSE) in the vessels and other tissues. Recently, higher levels of endogenous H2S have been detected in the brain than in other organs. This suggests that H2S may have a neuromodulatory role [3].
Some of the neuroprotective effects of H2S can be attributed to hypometabolism and hypothermia. For example, Blackstone et al. [1] observed that inhalation of H2S decreased the brain metabolic rate in mice by more than 50 % within 5 min. The metabolic rate is also associated with reductions in body temperature. However, the applicability of murine models of metabolism to larger animals has been questioned [4–6]. Furthermore, all the aforementioned studies focused on either whole-body metabolism or core temperature. However, changes in systemic metabolism, including oxygen extraction after cardiac arrest (CA), may vary more dramatically than brain metabolism [7]. To predict neurological outcomes, brain metabolism, especially cerebral extraction of oxygen (CEO2), must be taken into account to a greater extent than systemic metabolism [8, 9]. However, studies of brain metabolism and the effects of H2S on brain metabolism are rare. The effects of H2S on neurological function after CA and resuscitation remain poorly understood.
The aim of this study was to investigate the effects of inhaled exogenous H2S on brain metabolism and neurological function in rabbits after CA and resuscitation.
Materials and methods
For detailed materials and methods, see the electronic supplementary material (ESM).
Animal surgical procedures
This study was approved by the Institutional Animal Care and Use Committee of Harbin Medical University in Harbin, Heilongjiang, China, and followed national guidelines for the treatment of animals. Adult male New Zealand white rabbits, weighing 2.5–3.0 kg, were used in this study. Food and water were available ad libitum until the morning of the experiment.
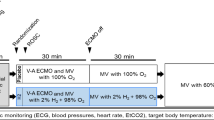
Anesthesia was performed with 1–2 % halothane and 30 % oxygen. Ventilation was controlled with a small animal ventilator (Kent Scientific, Litchfield, CT, USA) to maintain arterial pH at 7.35–7.45, PaCO2 at 35–45 mmHg, and PaO2 over 90 mmHg. All animals were mechanically ventilated except during the asphyxial CA procedures. Rectal temperatures were monitored continuously with thermal probes (BIOPAC Systems Inc., Santa Barbara, CA, USA) and the ambient temperature of 25 °C was maintained throughout the experiment. A standard lead II ECG was recorded continuously using subdermal needle-type electrodes placed in the limbs. All of the physiology data were monitored and recorded using the BIOPAC MP150 physiometer (BIOPAC Systems Inc., Santa Barbara, CA, USA).
Two saline-filled 18-gauge polyethylene catheters were inserted into the carotid artery for blood pressure measurement and into the left femoral vein for infusion and drug administration. Another 18-gauge catheter was placed in the right internal jugular vein and advanced in the retrograde direction until increased resistance was met at the base of the skull. The catheter was then withdrawn a few millimeters and secured, after which the blood flowed readily. The position of the catheter tip was at the right internal jugular bulb and confirmed by autopsy in animals that failed resuscitative efforts.
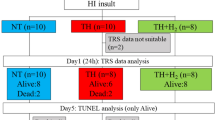
After preparation and subsequent stabilization, the animals were randomly assigned to four groups: sham group, sham/H2S group, CA group, and CA/H2S group (details in ESM).
Cardiac arrest and resuscitation
Cardiac arrest was induced in CA and CA/H2S groups by asphyxia as previously described [10, 11]. Asphyxia was induced by administering an additional dose of vecuronium and clamping the endotracheal tube. The cardiac arrest period was considered to begin when MAP decreased to less than 10 mmHg and left untreated for 3 min. Total asphyxia time was approximately 8 min. A detailed description of this model including the exact resuscitative procedures is provided in the ESM.
Measurement of brain temperature
Two digital electronic thermometers with thermocouple probes were inserted for continuous monitoring of the cortex and hippocampus temperature (details in ESM).
Determination of S100B level
Blood samples were draw from the internal jugular venous bulb and centrifuged for measuring the concentrations of S100B with a sandwich ELISA kit (details in ESM).
Neurological assessment and survival rate
All animals were evaluated before the experiment to ensure normal neurological function. Neurological dysfunction was evaluated daily in surviving animals with a clinical score previously validated in rabbits (0–10 % normal, 100 % brain death) [12]. Neurological dysfunction score (NDS) and the survival rates were assessed by the same investigator who was unaware of the group assignment during the 7 days after return of spontaneous circulation (ROSC).
Neuronal counts
On the 7th day after ROSC, the rabbits were deeply anesthetized with halothane. Hippocampi were removed and sectioned to 3 μm for hematoxylin and eosin staining. The numbers of viable neurons were counted in the CA1 region per high-power field (×400) (details in ESM).
Concentration of hydrogen sulfide
The concentrations of H2S in the plasma at baseline (t0), 30 min after ROSC (t1), and 60 min after ROSC (t2) were measured according to the method described previously (details in ESM) [13].
Measurement of brain metabolism
Cerebral extraction of oxygen (CEO2), arterio-jugular venous differences of glucose [AJVD(glu)], and lactate clearance were measured at t0, t1, and t2. A detailed description of brain metabolism is provided in the ESM.
Statistical analysis
Data are presented as mean ± SD. Intergroup differences in temperature, H2S concentration, CEO2, AJVD(glu), lactate, and S100B were assessed by two-way repeated-measures analysis of variance (ANOVA) with Bonferroni post hoc test. Differences in lactate clearance and neuron numbers were analyzed by one-way ANOVA with Bonferroni correction for post hoc comparison between multiple experimental groups. NDSs were compared with those of the corresponding CA group by use of a Mann–Whitney nonparametric test. Kaplan–Meier survival curves were compared using log-rank test. Significance was considered at the level of P < 0.05. Statistical analysis was performed using SPSS® software (SPSS Inc., Chicago, IL, USA).
Results
Forty-five rabbits were included in the present study. Twelve of 15 rabbits were successfully resuscitated in the CA and CA/H2S groups. The characteristics of CA and resuscitation showed no statistical differences between the CA and CA/H2S groups (ESM Table 1). There were no significant differences in physiological variables among all groups at the baseline period (ESM Table 2).
Rectal and cerebral temperature
Although the temperature was slightly lower in the H2S treated animals, no statistical differences were observed in rectal, cortex, and hippocampus temperatures between CA and CA/H2S groups during the 60 min after ROSC. There were also no significant differences in rectal, cortex, and hippocampus temperature between sham and sham/H2S groups (Fig. 1).
S100B
There was no significant difference in S100B between sham and sham/H2S groups. The level of serum S100B was significantly higher in the CA group than in the sham group at t1 (9.69 ± 1.50 vs. 7.00 ± 1.06 ng/ml, P < 0.05) and t2 (14.16 ± 1.99 vs. 6.99 ± 0.97 ng/ml, P < 0.05). However, this increase was significantly less pronounced in the CA/H2S group at t1 (8.63 ± 1.19 ng/ml) and t2 (10.85 ± 1.44 ng/ml) than in the CA group (both P < 0.05) (Fig. 2).
NDS and overall survival rate after ROSC
A significant difference in survival rate was shown between the CA/H2S group and CA group (P < 0.05) (Fig. 3a). At the end of the follow-up, five rabbits [42 % (5 of 12)] in the CA group and 10 rabbits [83 % (10 of 12)] in the CA/H2S group survived until day 7. It is noteworthy that the deaths occurred primarily between days 2 and 4. This is a time when death due to CA causes is uncommon and neurological causes predominate.
a Kaplan–Meier survival curves in the different experimental groups submitted to cardiac arrest and resuscitation. b Neurological dysfunction scores at day 1 after resuscitation in the different experimental groups. Open circles represent individual scores; thick line the median value of the corresponding group. # P < 0.05 versus CA group
As shown in Fig. 3b, at 24 h after ROSC, the NDS was significantly attenuated in the CA/H2S group compared with the CA group (P < 0.05) (Fig. 3b).
Neuronal counts
On the 7th day after CA and resuscitation, the neuronal density of the CA group was much lower than that of the sham group (Fig. 4a). The pyramidal neurons appeared swollen and arranged in irregular patterns. Nuclear pyknosis, karyorrhexis, and vacuolization were also observed. However, neuronal density and cell morphology were well preserved in the CA/H2S group. For comparison, the numbers of viable neurons per high-power field (×400) in the CA and CA/H2S groups were significantly lower than that of the sham group (both P < 0.05). The hippocampal CA1 counts were 24 ± 6 in the CA/H2S group, which was significantly more than in the CA group (10 ± 3, P < 0.05) but still less than in the sham group (39 ± 6, P < 0.05). No significant differences in CA1 neuron counts were observed between the sham and sham/H2S groups (Fig. 4b).
a Representative histological images of the hippocampus CA1 region from sham, sham/H2S, CA, and CA/H2S groups at 7 days after restoration of spontaneous circulation. All images were captured at ×400 magnification. Scale bar indicates 100 μm. b Number of viable neurons in the CA1 region belonging to different groups. *P < 0.05 versus sham group. # P < 0.05 versus CA group
H2S concentration
As can be seen in Fig. 5, the concentration of H2S was significantly increased after inhalation of exogenous H2S in the sham/H2S group at t1 and t2 (3.26 ± 0.47 and 3.74 ± 0.29 μmol/L, respectively) compared with the sham group (2.29 ± 0.07, 2.38 ± 0.14 μmol/L, respectively, both P < 0.05). These changes were also observed between the CA and CA/H2S groups at t1 (2.63 ± 0.33 vs. 6.43 ± 0.51 μmol/L) and t2 (2.92 ± 0.41 vs. 8.16 ± 0.38 μmol/L) (both P < 0.05). We also observed higher concentrations of H2S in the CA group relative to the sham group at t1 and t2. This difference was significant at t2 (P < 0.05). The levels of H2S with H2S therapy were significantly higher in animals undergoing CA compared to shams (P < 0.05 for both t1 and t2).
Brain metabolic measures
CEO2
As presented in Fig. 6, the levels of CEO2 at t1 were 16.72 ± 6.12 % in the CA group and 25.04 ± 7.11 % in the CA/H2S group. These were both significantly higher than those of the sham group (8.46 ± 4.73 %, both P < 0.05). This tendency was still observable at t2 (19.69 ± 6.02, 22.36 ± 7.70, and 9.31 ± 3.29 %, respectively, P < 0.05). The CA/H2S group had a higher level of CEO2 than the CA group at t1 (P < 0.05) and t2. No significant differences were observed between the sham and sham/H2S groups throughout the 60 min of the experiment.
Cerebral extraction of oxygen, arterio-jugular venous differences of glucose, and lactate in sham, sham/H2S, CA, and CA/H2S groups. Lactate clearance shows the clearance of lactate between t0 and t1 [Lactate (t0–t1)], between t0 and t2 [Lactate (t0–t2)], and between t1 and t2 [Lactate (t1–t2)] for each group. T0, T1, and T2 represent baseline, 30 and 60 min after restoration of spontaneous circulation. *P < 0.05 versus sham group. # P < 0.05 versus CA group
Arterio-jugular venous differences in glucose concentration
The values of AJVD(glu) were significantly higher in the CA group (1.18 ± 0.38 mmol/L, P < 0.05) but only slightly higher in the CA/H2S group (0.77 ± 0.29 mmol/L) than sham values (0.44 ± 0.14 mmol/L) at t1. There was a significant decrease in AJVD(glu) in the CA/H2S group compared with the CA group at t1 (P < 0.05). The increase in AJVD(glu) in the CA group returned to normal values at t2 (Fig. 6). No significant differences in AJVD(glu) were observed between the sham and sham/H2S groups at any time.
Lactate and lactate clearance
A statistically significantly higher lactate level was observed in the CA (6.01 ± 0.64 mmol/L) and CA/H2S group (5.11 ± 0.43 mmol/L) at t1 than in the sham group (3.50 ± 0.33 mmol/L, both P < 0.05). Differences in lactate levels between the CA and CA/H2S groups reached statistical significance at t1 (P < 0.05) and widened by t2 (6.11 ± 0.77 vs. 3.83 ± 0.56 mmol/L, P < 0.05) (Fig. 6).
After ROSC, lactate clearance of Lactate (t0–t1) and Lactate (t0–t2) were negative, indicating an increase in lactate level. However, in the CA/H2S group, Lactate (t1–t2) was positive, indicating lactate clearance after ROSC. As shown in Fig. 6, the values of Lactate (t0–t1) (74.22 ± 12.59 %) and Lactate (t0–t2) (77.04 ± 28.20 %) were significantly higher in the CA group than in the sham group (0.48 ± 5.76, 3.49 ± 6.93 %, both P < 0.05). However, Lactate (t0–t1) (45.98 ± 13.43 %) and Lactate (t0–t2) (9.72 ± 19.67 %) were significantly lower in CA/H2S group than in the CA group (both P < 0.05).
Discussion
The current study investigated the effects of exogenous H2S on cerebral metabolism and neurological function following CA and resuscitation. The key findings were that H2S reduced neurohistopathological damage and improved early neurological function after CA and resuscitation. Increased CEO2, decreased AJVD(glu), and enhanced lactate clearance were observed at the same time.
In this study, we observed that inhaling exogenous 80 ppm H2S gas significantly increased the concentration of H2S in plasma. Interestingly, we observed an increase of H2S after ROSC even without inhalation of exogenous H2S in the CA group. Several researchers have described significant changes of H2S in plasma levels in various disease states [14–16]. A recent study with cerebellar slices and intact mouse brains identified that during hypoxia inhibition of heme oxygenase 2 mediated CO production, with a corresponding release of the tonic inhibition of CBS, and allowing CBS to generate H2S [17]. On the other hand, the decreased capacity of rhodanese, which is the key component in the H2S removal system under low O2 tensions, may effectively increase the H2S concentrations [18]. Eto and colleagues [2] found that the excitatory neurotransmitter l-glutamate greatly enhanced the production of H2S. And many studies have confirmed that there is a massive outpouring of extracellular glutamate after CA or cerebral ischemia and reperfusion injury [19, 20]. On the basis of these studies we hypothesize that hypoxia and glutamate excess drive increased post-ischemic H2S levels and the synergistic increase with H2S therapy.
Much research has been published on the clinical utility of S100B in predicting neurological outcomes after CPR [21, 22]. In this study, we found that serum S100B levels were significantly increased after ROSC, whereas inhalation of H2S significantly reduced the level of S100B, which predicted a better neurological outcome. This was confirmed by the results of NDS. The 24-h NDS data, when survivors were nearly equal between CA and CA/H2S groups, clearly showed less neurological injury in H2S treated animals. And this protective effect continued throughout the experiment, which was proven by the increase in viable neurons and survival at 7 days after ROSC. Taking all these factors together, the present data provide strong evidence that H2S can improve early neurological outcomes after CA and resuscitation.
Although decreased body temperature and metabolism were observed in mice exposed to H2S [1, 23], these results had been questioned in piglets [4], sheep [5], and even rats [6]. Similarly in our rabbit model, rectal, cortex, and hippocampus temperatures were not decreased significantly in H2S treated animals. Owing to their large surface area/mass ratio, mice can experience rapid drops in core body temperature, whereas these are rare or impossible in larger animals. Another key factor was the low O2 tension which was indispensable for H2S-dependent hypometabolism [6]. Stein and colleagues [6] found that rats were largely insensitive to H2S-induced low body temperature unless O2 tension was decreased to 10.5 %. This finding was supported by our study with temperature results during the short periods of exposure to lowered O2 tensions.
In the present study, we observed an increased CEO2 after CA which is compatible with previous studies [24]. However, Lemiale and colleagues [25] found that CEO2 reached nearly normal values over time in survivors. These different results of CEO2 could probably be explained in part by the therapeutic hypothermia and midazolam used for sedation in Lemiale’s study which can depress the brain metabolism. Another explanation could be the different degree of brain damage. In Lemiale’s study, more severe brain damage was found in 10 of 18 patients and nearly half of them had serious seizures [25]. More severe brain damage meant extensive loss of viable neurons with a concomitant decrease in cerebral metabolism [24, 26].
The current findings also demonstrate that less glucose extraction and faster lactate clearance occur after inhalation of H2S. Taking into account the higher CEO2, one possibility could be a switch from anaerobic glycolysis induced by asphyxia to oxidative phosphorylations which consume more oxygen and less glucose while producing less lactate. In the latest study [27], evidence had shown that H2S could correct the pathophysiological switch between oxidative phosphorylation and glycolysis, which gave a powerful support to our study.
Many studies demonstrated that the increased CEO2 [25, 26] or lower CJVO2 (content of oxygen in the jugular venous blood) [24, 28] and effective early lactate clearance were associated with improvement of neurological outcome and decreases in both early and late mortality in post-CA patients [29, 30]. Consistent with these findings, a better neurological outcome was confirmed with less S100B, more viable neurons, less NDS, and higher survival rate in H2S treated animals which had increased CEO2, decreased AJVD(glu), and enhanced lactate clearance.
This study has several limitations. Firstly, we used 80 ppm H2S because this dose had been widely used in previous studies and no obvious neurotoxic effects were observed [1, 4, 31]. Although this will make it easier to draw comparisons, the relationship between H2S concentration and favorable outcome has not been verified. Furthermore, the lack of measurement of CBF and ATP generation/ox-phos limits our ability to draw a conclusion whether the brain metabolism changes are related to delivery or consumption. Future studies are warranted to further investigate the relationship between H2S effects and CBF and ATP generation/ox-phos. Lastly, though our findings demonstrate improvement of early neurological function after inhalation of 80 ppm H2S in a rabbit CA model, the translational implication of H2S in the human clinical setting is uncertain and needs to be investigated.
In summary, our study revealed that inhalation of 80 ppm H2S reduced neurohistopathological damage and improved early neurological function after CA and resuscitation in rabbits. We suggest that improvements in cerebral metabolism evidenced by increasing CEO2, decreasing AJVD(glu), and clearing lactate may be involved in the protective effects. A better understanding of the relationship between H2S effects and CBF and ATP generation/ox-phos in future studies will be important in clarifying the underlying mechanisms.
References
Blackstone E, Morrison M, Roth MB (2005) H2S induces a suspended animation-like state in mice. Science 308:518
Eto K, Ogasawara M, Umemura K, Nagai Y, Kimura H (2002) Hydrogen sulfide is produced in response to neuronal excitation. J Neurosci 22:3386–3391
Szabó C (2007) Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 6:917–935
Li J, Zhang G, Cai S, Redington AN (2008) Effect of inhaled hydrogen sulfide on metabolic responses in anesthetized, paralyzed, and mechanically ventilated piglets. Pediatr Crit Care Med 9:110–112
Haouzi P, Notet V, Chenuel B, Chalon B, Sponne I, Ogier V, Bihain B (2008) H2S induced hypometabolism in mice is missing in sedated sheep. Respir Physiol Neurobiol 160:109–115
Stein A, Mao Z, Morrison JP, Fanucchi MV, Postlethwait EM, Patel RP, Kraus DW, Doeller JE, Bailey SM (2012) Metabolic and cardiac signaling effects of inhaled hydrogen sulfide and low oxygen in male rats. J Appl Physiol 112:1659–1669
Müllner M, Sterz F, Domanovits H, Zeiner A, Laggner AN (1996) Systemic and cerebral oxygen extraction after human cardiac arrest. Eur J Emerg Med 3:19–24
Cruz J, Nakayama P, Imamura JH, Rosenfeld KG, de Souza HS, Giorgetti GV (2002) Cerebral extraction of oxygen and intracranial hypertension in severe, acute, pediatric brain trauma: preliminary novel management strategies. Neurosurgery 50:774–779
Cruz J (1996) Relationship between early patterns of cerebral extraction of oxygen and outcome from severe acute traumatic brain swelling: cerebral ischemia or cerebral viability? Crit Care Med 24:953–956
Chen MH, Xie L, Liu TW, Song FQ, He T, Zeng ZY, Mo SR (2007) Epinephrine, but not vasopressin, improves survival rates in an adult rabbit model of asphyxia cardiac arrest. Am J Emerg Med 25:509–514
Hickey RW, Kochanek PM, Ferimer H, Alexander HL, Garman RH, Graham SH (2003) Induced hyperthermia exacerbates neurologic neuronal histologic damage after asphyxial cardiac arrest in rats. Crit Care Med 31:531–535
Baker AJ, Zornow MH, Grafe MR, Scheller MS, Skilling SR, Smullin DH, Larson AA (1991) Hypothermia prevents ischemia-induced increases in hippocampal glycine concentrations in rabbits. Stroke 22:666–673
Ang SF, Moochhala SM, Bhatia M (2010) Hydrogen sulfide promotes transient receptor potential vanilloid 1-mediated neurogenic inflammation in polymicrobial sepsis. Crit Care Med 38:619–628
Bhatia M, Sidhapuriwala J, Moochhala SM, Moore PK (2005) Hydrogen sulphide is a mediator of carrageenan-induced hindpaw oedema in the rat. Br J Pharmacol 145:141–144
Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, Moore PK (2005) Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J 19:623–625
Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, Moore PK (2005) Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J 19:1196–1198
Morikawa T, Kajimura M, Nakamura T, Hishiki T, Nakanishi T, Yukutake Y, Nagahata Y, Ishikawa M, Hattori K, Takenouchi T, Takahashi T, Ishii I, Matsubara K, Kabe Y, Uchiyama S, Nagata E, Gadalla MM, Snyder SH, Suematsu M (2012) Hypoxic regulation of the cerebral microcirculation is mediated by a carbon monoxide-sensitive hydrogen sulfide pathway. Proc Natl Acad Sci U S A 109(4):1293–1298
Stein A, Mao Z, Morrison JP, Fanucchi MV, Postlethwait EM, Patel RP, Kraus DW, Doeller JE, Bailey SM (2012) Metabolic and cardiac signaling effects of inhaled hydrogen sulfide and low oxygen in male rats. J Appl Physiol 112(10):1659–1669
Lau A, Tymianski M (2010) Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 460:525–542
Tseng EE, Brock MV, Lange MS, Troncoso JC, Blue ME, Lowenstein CJ, Johnston MV, Baumgartner WA (2010) Glutamate excitotoxicity mediates neuronal apoptosis after hypothermic circulatory arrest. Ann Thorac Surg 89:440–445
Shinozaki K, Oda S, Sadahiro T, Nakamura M, Hirayama Y, Abe R, Tateishi Y, Hattori N, Shimada T, Hirasawa H (2009) S-100B and neuron-specific enolase as predictors of neurological outcome in patients after cardiac arrest and return of spontaneous circulation: a systematic review. Crit Care 13:R121
Grubb NR, Simpson C, Sherwood RA, Abraha HD, Cobbe SM, O’Carroll RE, Deary I, Fox KA (2007) Prediction of cognitive dysfunction after resuscitation from out-of-hospital cardiac arrest using serum neuron-specific enolase and protein S-100. Heart 93:1268–1273
Nicholson RA, Roth SH, Zhang A, Zheng J, Brookes J, Skrajny B, Bennington R (1998) Inhibition of respiratory and bioenergetic mechanisms by hydrogen sulfide in mammalian brain. J Toxicol Environ Health A 54:491–507
Buunk G, van der Hoeven JG, Meinders AE (1999) Prognostic significance of the difference between mixed venous and jugular bulb oxygen saturation in comatose patients resuscitated from a cardiac arrest. Resuscitation 41(3):257–262
Lemiale V, Huet O, Vigué B, Mathonnet A, Spaulding C, Mira JP, Carli P, Duranteau J, Cariou A (2008) Changes in cerebral blood flow and oxygen extraction during post-resuscitation syndrome. Resuscitation 76:17–24
Müllner M, Sterz F, Domanovits H, Zeiner A, Laggner AN (1996) Systemic and cerebral oxygen extraction after human cardiac arrest. Eur J Emerg Med 3:19–24
Suzuki K, Olah G, Modis K, Coletta C, Kulp G, Gerö D, Szoleczky P, Chang T, Zhou Z, Wu L, Wang R, Papapetropoulos A, Szabo C (2011) Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc Natl Acad Sci U S A 108:13829–13834
Takasu A, Yagi K, Ishihara S, Okada Y (1995) Combined continuous monitoring of systemic and cerebral oxygen metabolism after cardiac arrest. Resuscitation 29:189–194
Kliegel A, Losert H, Sterz F, Holzer M, Zeiner A, Havel C, Laggner AN (2004) Serial lactate determinations for prediction of outcome after cardiac arrest. Medicine (Baltimore) 83:274–279
Donnino MW, Miller J, Goyal N, Loomba M, Sankey SS, Dolcourt B, Sherwin R, Otero R, Wira C (2007) Effective lactate clearance is associated with improved outcome in post-cardiac arrest patients. Resuscitation 75:229–234
Volpato GP, Searles R, Yu B, Scherrer-Crosbie M, Bloch KD, Ichinose F, Zapol WM (2008) Inhaled hydrogen sulfide: a rapidly reversible inhibitor of cardiac and metabolic function in the mouse. Anesthesiology 108:659–668
Acknowledgments
The study was supported by grants from the National Natural Science Foundation of China (81000822) and the Natural Science Foundation of Heilongjiang Province, Heilongjiang, China (QC2010056). This work was assisted in part by the Key Laboratory of Anesthesiology and Intensive Care Research of Heilongjiang Province, China.
Conflicts of interest
There was no conflict of interest in this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wei, X., Duan, L., Bai, L. et al. Effects of exogenous hydrogen sulfide on brain metabolism and early neurological function in rabbits after cardiac arrest. Intensive Care Med 38, 1877–1885 (2012). https://doi.org/10.1007/s00134-012-2714-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2714-x