Article Text
Statistics from Altmetric.com
Case presentation
A 32-year-old-woman presented with traumatic facial injuries from a horseback riding incident. She was found to have a Glasgow Coma Score of 3T, left eyelid ptosis with a fixed-dilated pupil and a flaccid right upper extremity. CT angiogram of the head showed complex craniofacial injuries, including a transverse fracture transecting the clivus and the left petrous temporal bone, with SAH in the suprasellar cistern. There was a bilateral engorgement of the superior ophthalmic veins, suggestive of bilateral CCF formations. Digital subtraction angiography (DSA) showed a left petrocavernous internal carotid artery dissection (ICAD). MRI of the brain demonstrated multiple embolic infarcts in the left middle cerebral artery (MCA) territory, despite therapeutic anticoagulation with heparin. Follow-up DSA 1 week later showed bilateral multifocal internal carotid artery (ICA) and vertebral artery dissections, bilateral direct CCFs and cavernous ICA PAs. Because of progressing morphological distortion (figure 1), a flexible LVIS Jr. stent was placed at the anterior genu of the left cavernous ICA to provide a scaffold for the more rigid 3.5×20.0 mm self-expanding Wingspan stent (petrous ICA to distal cavernous ICA), covering the neck of the PA followed by its coil embolisation. Five days after coiling, a 5 mm residual sac was noted in the PA with extension of the left ICAD up to the ICA terminus (figure 2). The latter was treated with another 3.5×23.0 mm LVIS Jr. stent (from left the M1 MCA up to the dural junction of the left ICA). Eleven days after coiling, the PA had increased up to 15×14 mm and was treated with three PED constructs deployed in a pseudo-telescoping manner from the petrous ICA to the supraclinoid ICA causing immediate flow diversion (figure 3). While maintained on a heparin drip during the procedure, she was transitioned to eptifibatide and aspirin after stent placement, and then on DAPT (aspirin (81 mg) and clopidogrel (75 mg) daily) 4 weeks from initial stenting.
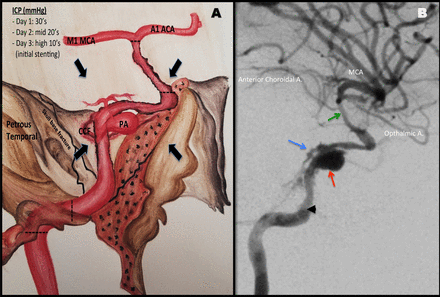
(A) Schematic representation of the post-traumatic ICAD suffering external compression (black arrows) from skull-base fractures and cerebral oedema from coexisting ischaemia/traumatic brain injury resulting in high ICPs. (B) Third digital subtraction angiography (1 week from injury), lateral view: left ICAD extending from the precavernous ICA (arrowhead) to the supraclinoid segment (green arrow) with a 7 mm dissecting PA directed anterosuperiorly (red arrow) at the junction of the petrous and precavernous ICA with contrast percolation from the posterior genu forming a direct CCF (blue arrow). A., artery; ACA, anterior cerebral artery; CCF, carotid cavernous fistula; ICA, internal carotid artery; ICAD, intracranial carotid artery dissection; ICP, intracranial pressure; MCA, middle cerebral artery; PA, pseudoaneurysm.
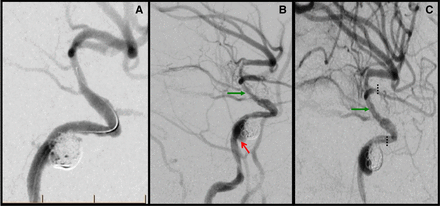
(A) Fourth DSA (8 days from injury), lateral view: coil embolisation of the PA achieved after placement of a Wingspan stent (from the petrous ICA to the distal cavernous ICA) through a scaffold created by placing an LVIS Jr. stent at the anterior genu. (B) Fifth DSA (5 days after coiling), lateral view: 5 mm residual sac developing along the medial aspect of the coiled PA (red arrow) with extension of the left intracranial carotid artery dissection up to the ICA terminus (green arrow). (C) Postdeployment of a 3.5×23.0 mm LVIS Jr. stent (dotted line) placed from the left M1 middle cerebral artery up to the dural junction of the left ICA (green arrow). DSA, digital subtraction angiography; ICA, internal carotid artery; PA, pseudoaneurysm.
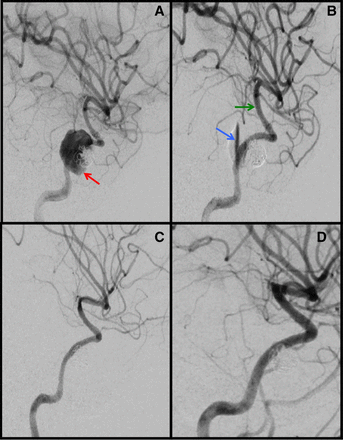
(A) Sixth DSA (11 days from coiling), lateral view: coiled dissecting PA now measuring 15×14 mm (red arrow) treated with three PED constructs with immediate flow diversion (blue arrow), with retained luminal patency of the supraclinoid ICA (green arrow) (B). Follow-up DSA at 12 months (C) and 24 months (D) from injury showing retained resolution of the intracranial carotid artery dissection–PA–carotid cavernous fistula complex. DSA, digital subtraction angiography; PA, pseudoaneurysm.
Outcome and follow-up
Follow-up DSA at 12 and 24 months after PED placement showed a normal calibre of the left ICA with retained occlusion of the PA and CCF (figure 3). Neurological exam at 18-month follow-up showed minimal transcortical motor aphasia, partial right cranial nerve III/VI palsy and moderate executive dysfunction on neuropsychological evaluation.
Discussion
Blunt trauma-induced formation of an ICAD–PA–CCF complex is rare. Such PAs have a mortality rate of ~32% to 50%, rarely regressing spontaneously with a high incidence of rupture, typically within the first 3 weeks.2 Treatment options include (1) PA clipping (not preferred due to PA wall friability), (2) parent vessel sacrifice or (3) endovascular coiling and stenting. Cohen et al described two patients with an ICAD–PA–CCF complex following blunt trauma, successfully treated with stent-assisted coiling in one and covered stents in another.3 Lim et al described a ruptured traumatic PA of supraclinoid ICA treated successfully with a stent-in-stent technique in the acute SAH period (using DAPT before and after the procedure).4 Prasad et al reported the first successfully treated case of traumatic multifocal ICAD–ruptured PA–CCF complex using a PED (7 months after two prior coil embolisations failed).5 While PEDs have become popular for vessel reconstruction, their limitations in such cases include (1) SAH (given the need for DAPT), (2) concern for the bulky delivery system further damaging already injured vessels and (3) PA rerupture (as demonstrated by Kadkhodayan et al).6 Thus, such cases are often managed via parent vessel sacrifice, which caries a 4.7% risk of stroke even if adequate collateral flow is noted during the balloon occlusion test, supporting the argument for parent vessel preservation.7
To the best of our knowledge, this is the first case describing a staged endovascular approach to treating an ICAD–ruptured PA–CCF complex. As seen in our patient, the resulting stenosis from post-traumatic ICADs can be exacerbated by external compression (figure 1). To stabilise the angioarchitecture, we placed a self-expanding Wingspan stent because of its high radial force, which would help combat the high intracranial pressure. However, with extension of the ICAD and recurrence of the PA 18 days from presentation, two PEDs were deployed, overlying the Wingspan stent covering the neck of the PA with a third PED telescoped within. The initial SAH was a concern, given the need for DAPT; however, multiple authors have noted the bleeding risk with DAPT after stenting to be related to ventricular catheter placement/revision, rather than aneurysmal bleeding.8
Finally, our case provides anecdotal evidence that PEDs can endothelialise even when layered on top of other stents (one layer of PED, another layer of Wingspan and a third layer of LVIS Jr. at the anterior genu), with DAPT for the first 12 months followed by aspirin monotherapy.
In conclusion, early stabilisation of the intracranial angioarchitecture with a combination of rigid and flexible stents, with eventual vessel reconstruction with FD stents, may be an alternative approach to deconstructive procedures in the treatment of a post-traumatic ICAD–PA–CCF complex.
Footnotes
Contributors KKT: manuscript conception, design, writing and editing, and clinical care of the patient. MH and JM: manuscript design, final review of the article and clinical care of the patient. IK, AN, MP and BK: critical review of the manuscript and clinical care of the patient. DL: manuscript conception, design, critical review of the manuscript and clinical care of the patient.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.