Article Text
Abstract
Advances in endovascular treatment of acute ischaemic stroke from intracranial large vessel occlusions have continued in the past decade. Here, we performed a detailed review of all the new trials and studies that had the highest evidence, the guidelines for mechanical thrombectomy, the selection of the particular population outside the guidelines and endovascular therapeutic strategies for acute ischemic stroke from occluded intracranial arteries.
- atherosclerosis
- intervention
- stroke
- thrombectomy
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Stroke remains the second-leading cause of death and leading cause of the disability-adjusted life-years (DALYs) globally.1 In China, stroke and ischaemic heart disease were the leading causes of death and DALYs in 2017.2 Ischaemic stroke accounted for 71% of all strokes globally and 81.9% in China, of which 20% were acute ischaemic stroke (AIS) from large vessel occlusions (LVOs).3 With technological advances in new thrombectomy devices and interventional therapy, endovascular thrombectomy (EVT) with stent retrievers and/or reperfusion catheters has recently become the standard treatment for AIS-LVO of the anterior circulation.4
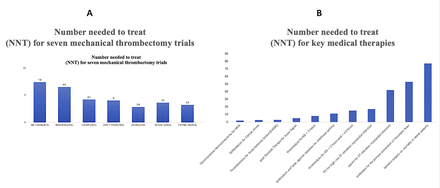
The number needed to treat (NNT) to achieve a 90-day functional independence of patients with AIS-LVO from the mechanical thrombectomy (MT) was 2.8–7.4 (figure 1), while the NNT from medical therapies was higher (figure 1).5 6 It is estimated that about 30% of AISs are due to LVO in the USA, and that 30–40 pts/100 000/year have clot locations (ICA/M-1, M-2, basilar) eligible for EVT.7 8 About 35%–40% of all AIS were from a proximal LVO in China, but only 28.1‱ of patients with AIS-LVO received intracranial intra-arterial thrombectomy according to the data from the Hospital Quality Monitoring System in 2018.9 This major gap remains a challenge and suboptimal for those patients with AIS who may benefit. Therefore, a more practical and evidence-based AIS-LVO thrombectomy system needs to be established in the new era.
The NNT for major RCTs of mechanical thrombectomy and other key medical therapies.5 (A) Illustrating the NNT for seven RCTs. (B) The NNT for key medical therapies including decompressive haemicraniectomy for MCA infarction, defibrillation for cardiac arrest, thrombectomy for AIS (the DAWN Trial), goal-directed therapy for acute sepsis, thrombolysis for AIS <3 hours, ipratropium and beta-agonist nebulizer for childhood asthma, thrombolysis for AIS >3 hours and <4.5 hours, PCI for high-risk ST-elevation myocardial infarction, aspirin for ST-elevation myocardial infarction, antibiotics for the primary prevention of rheumatic fever and bariatric surgery on mortality in obese patients. AIS, acute ischaemic stroke; MCA, middle cerebral artery; NNT, number needed to treat; PCI, percutaneous coronary intervention; RCTs, randomised controlled trials.
MT performed within guidelines
Both 2018 and 2019 editions of the Guidelines for the Early Management of Patients with Acute Ischemic Stroke by AHA/ASA provided the recommendation (Grade IA evidence) that patients with AIS-LVO should receive endovascular treatment when presented within 0–6 hours of onset (Grade IA evidence). Patients with AIS-LVO should receive MT if they meet all of the following criteria: (1) prestroke modified Rankin Scale (mRS) score of 0–1; (2) causative occlusion of the internal carotid artery or middle cerebral artery (MCA) segment 1 (M1); (3) age ≥18 years; (4) NIHSS (National Institute of Health stroke scale) score of ≥6; (5) Alberta Stroke Program Early CT Score (ASPECTS) of ≥6; and (6) treatment can be initiated (groin puncture) within 6 hours of symptom onset (figure 2).10 In addition, patients with AIS-LVO presented between 6 and 16 hours with a neuroimaging finding of a mismatch (the mismatch defines a smaller infarct core with a larger ischaemic penumbra in the DEFUSE 3 trial (Endovascular Therapy Following Imaging Evaluation forIschemic Stroke)) (Grade IIa recommendation, Grade B-R evidence) should receive endovascular treatment.
Triage algorithm for AIS with LVO. AIS, acute ischaemic stroke; ASPECTS, AlbertaStroke Program Early CT Score; LVO, largevessel occlusion; mRS, modifiedRankin Scale.
The stent retriever has been the first-line thrombectomy device used in five randomised controlled trials (RCTs) treating patients with AIS-LVO within 6 hours of onset (MR CLEAN,11 ESCAPE,12 REVASCAT,13SWIFT PRIME14 and EXTEND IA,15 and also in DAWN16 and DEFUSE 317 trials that treated patients with AIS-LVO between 6 and 24 hours of onset). A major revision in the 2019 update of the guideline was that the use of aspiration was not inferior to stent retriever thrombectomy as a first-line approach for AIS-LVO (Grade I recommendation, B-R grade Evidence).18–21
Shortening onset to puncture time may improve outcome
We also reviewed recent real-world or observative studies, single-centre or multicentre trials in detail to discuss MT performed beyond the guideline recommendations.
Direct transfer to angiosuite
EVT is strongly time-dependent, with 20% decreased probability of 90-day functional independence for each 1-hour delay to reperfusion.22 Minimise the delay from the onset-to-puncture time is the first step of endovascular procedure for patients with AIS-LVO.23 24 Previous retrospective observational studies and a case-controlled study suggested that the median door-to-groin time and onset-to-groin times were significantly shorter if an patient with AIS-LVO was directly transferred to an angiosuite (DAS).25–27 A recent report demonstrated improved outcomes but with only 13 min faster onset to puncture.28 Moreover, DAS will lead to patients being taken for catheter arteriography who do not have LVO and requires an angiography room and team to be rapidly available, which may not be possible in many hospitals.
Additional WE-TRUST trial (Workflow Optimization to Reduce Time to Endovascular Reperfusion for Ultra-fast Stroke Treatment, NCT04701684) will be running in 16 sites to enrol 506 patients globally from February 2021 to December 2023. For patients with AIS-LVO with early onset of symptoms (≤6 hours), skipping multimode CT or MR could significantly reduce the workflow time and optimise patient preparation before surgery (figure 2).
Patients selection
Large-core infarction
Most previous RCTs excluded patients with AIS-LVO with an ASPECTS <6. The previous Hermes study pooled five RCTs of MT and compared with the Best Medical Management (BMM). A favourable outcome from MT for larger cores could not be established.22 The recent SELECT trial enrolled patients with a large-core infarction in the anterior circulation, or an ASPECTS of <5 on a non-contrast CT, or an ischaemic core volume of >50 cm3 on CTP, or a relative cerebral blood flow of <30% at presentation.29 This prospective cohort study indicated that patients had a favourable outcome (MT 31% vs BMM 14%, p=0.03), if their ASPECTS were between 3 and 5 and an ischaemic core volume was of <100 cm3. These results have been incorporated into the design of SELECT2 trial and other RCTs of MT for patients with a large-core infarction (table 1).
Trials of endovascular thrombectomy for stroke with a large ischaemic core
Mild strokes
The decision to perform thrombectomy in patients with mild strokes (NIHSS 0–5) has been controversial. Analysis of a retrospective multicentre cohort study showed no improvement in functional independence in patients with AIS with mild stroke who underwent MT (63.3% MT vs 67.8% BMM), but rate of symptomatic intracranial haemorrhage (sICH) was increased (5.8% MT vs 0% BMM).30 Another meta-analysis that pooled four international multicentre studies found that MT was not superior to BMM for patients with AIS with mild strokes (NIHSS <6). In unadjusted analyses, MT was associated with higher odds of sICH.31 Seners et al indicated that the likelihood of early neurologic deterioration is correlated with more proximal and longer clot lengths, potentially allowing selection for EVT of those patients more like to deteriorate. They have noted that if a patient deteriorates and then undergoes rescue MT, the outcome is worse than expected for primary MT.32 Furthermore, the added value of MT may depend on whether or not the patient was able to receive intravenous alteplase, which may rapidly dissolve an LVO about 10% of the time,33 with greater success over longer time durations (up to 44.9% of clots <8 mm dissolved).34
Currently EXTREMIS-MOSTE (NCT04167527), Tempo-2 (NCT02398656) and ENDO-LOW Trials (NCT03796468) are testing a similar hypothesis of MT for patients with mild AIS-LVO.
The children and the elder
There is no Class IA evidence to support endovascular therapy in children with an AIS-LVO. A systematic review suggested that MT might be feasible and safe for paediatric patients with AIS-LVO (ICA terminus, M1, basilar artery).35 Due to the unique features of anatomy, imaging parameters, and aetiology of stroke in children, decision on treating children with an AIS-LVO with MT or not should be made by a multidisciplinary approach.36
Octogenarians were similarly underrepresented in major RCTs of EVT. However, more patients >80 years of age underwent MT from 2014 to 2016 (0.83% to 1.83%). In this age group, the rate of improved functional independence was low (9%), and the in-hospital mortality rate was high (19%).37 One multicentre, retrospective study showed that age ≥80 independently predicted a high rate of success in recanalisation, but a higher rate of postoperative ICH.38 Meta-analyses of the multiple randomised trials have shown that the elderly does have worse outcomes than younger patients but that the OR for benefit from MT is similar.22 Even non-agenarians seem to benefit of reperfusion is successful.39 Therefore, the current ESO/ESMINT recommendation is that age should not be used as an exclusion for MT in the elderly.40
Posterior circulation
Posterior circulation has these unique features: (1) the anatomy and variant of the posterior circulation vessels are often complex; (2) symptoms of posterior circulation stroke may be mild and difficult to identify; (3) symptoms may progress fast and increase the risks of disability and mortality; and (4) no adequate imaging tool to assess for its perfusion status.
Basilar Artery International Cooperation Study (BASICS) showed that intra-arterial therapy did not provide superior clinical outcomes over intravenous thrombolysis (IVT) for patients with basilar artery occlusion.41 The trial of endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST) was completed in China. The high crossover rate (13%) and progressive loss of equipoise of valid per-centre recruitment led to poor adherence to the designed trial. The trial was terminated early after recruiting 131 of the planned sample sizes of 344 participants. Consequently, there was no statistical significance in the primary endpoint (90-day mRS 0–3 score) between the in the MT group (32%) and control group (42%) in the intention-to-treat analysis.42
In 2020, a real-world no-nrandomised cohort study from 47 comprehensive stroke centres in China indicated that BMM plus MT substantially improved the 90-day functional outcomes (adjusted cOR: 3.08, p<0.001) and reduced the rate of 90-day mortality (adjusted cOR: 2.93, p<0.001). In May 2020, BASICS reported its results of treating patients with an acute basilar artery territory stroke via a webinar during the ESO-WSO Conference. MT of occluded basilar artery only improved by about 6.5% and did not achieve a significant difference.43 Basilar Artery Occlusion Chinese Endovascular Trial (NCT02737189) is currently ongoing.
Medium vessel occlusions (MEVOs)
Goyal et al proposed the anatomical definition of MEVO that M2/3, A2/3 or P2/3, in addition, the functional definition includes NIHSS ≥5 or NIHSS <5 with disabling deficit.44–46 These vessel occlusions are common but can be devastating clinically. Although emerging reports suggest that EVT may be safe and effective for MEVO, at present there is a lack of clear guideline recommendations.22 47 48
Tandem lesion
Tandem lesion indicates that extracranial internal carotid artery or vertebral artery stenosis/occlusion is associated with MCA or BA occlusion. The effect of EVT for AIS-LVO caused by the tandem lesion in anterior circulation was comparable to the non-tandem occlusion in the HERMES study.22 But the procedure commonly is technically more difficult, time-consuming, and maybe need ICA stenting which is associated with risk of sICH due to more antiplatelet agents.49 There is no statistical difference between the technique (the anterograde or retrograde method) used and the functional outcome. At present, EVT for tandem lesions has not been explicitly established as the standard of care.10
AIS-LVO in patients with COVID-19
COVID-19 pandemic has had an impact on overall stroke care globally.50 51 Based on the silent transmission of the novel COVID-19, patients with acute stroke concurrent with or without COVID-19 have been forcing us to increase the delays of the screen and care for patients.37 A consensus on prevention and management of COVID-19 for neurologists from China provided that the workflow and streamline in neurological emergency and staffing green pathway for acute stroke.50 52 A Germany nationwide database indicated that patients with AIS with COVID-19 had a lower rate of MT (with COVID-19, 3.8% vs without COVID-19, 7.9%), but a higher rate of in-hospital mortality (with COVID-19, 22.5% vs without COVID-19, 7.8%).53 European Multicenter Study of ET-COVID-19 also pointed out a higher rate of 30-day mortality (29%) after MT among patients with AIS with COVID-19.54 We need immediate consideration of a new streamline of MT for AIS-LVO with COVID-19 in the contexts of the pandemic evolution.52 55
Treatment strategies
Bridging therapy versus direct MT within 6 hours of onset of LVO
Bridging therapy is the standard of care in patients with AIS-LVO if presented within 6 hours of onset. However, it has been controversial if this patient population can be treated without being given intravenous tPA first. Data from five major randomised trials (MR CLEAN, ESCAPE, REVASCAT, SWIFT PRIME and EXTEND IA) were pooled by HERMES collaboration and showed no statistical difference in functional independence among the 1090 cases treated with bridging strategy and 188 cases with MT alone (OR: 2.45 vs 2.43, p=0.43).22 To settle this controversy, five trials compared direct MT without IVT in this patient population (table 2). Both SKIP (The Randomized Study of Endovascular Therapy With vs Without Intravenous Tissue Plasminogen Activator in Acute Stroke with ICA and M1 Occlusion) and DIRECT-MT (Direct Intraarterial Thrombectomy in Order to Revascularize Acute Ischemic Stroke Patients with Large Vessel Occlusion Efficiently in Chinese Tertiary Hospitals: A Multicenter Randomized Clinical Trial) trials were conducted in Asia and designed as non-inferiority studies. The SKIP trial from Japan failed to demonstrate the non-inferiority of direct MT to bridging therapy. Nevertheless, any intracranial haemorrhage was significantly lower in the direct MT group without intravenous tPA.56 SKIP used lower dose of tPA (0.6 mg/kg). While the DIRECT-MT trial was conducted from 41 academic care centres in China, and first confirmed that patients who underwent MT were non-inferior to the bridging therapy with a standard dose of tPA as the currently highest evidence. But this study might be shown that insufficient evidence for successful reperfusion of tPA alone because of tPA infusion in 296 of 319 cases completed during the endovascular procedure but not before MT. Because of these two trials of generous non-inferiority design, the CI did not exclude about 20% of the benefit in the bridging therapy group.57
Trials of direct mechanical thrombectomy or bridge treatment in an early time window
DEVT study (Effect of Endovascular Treatment Alone vs Intravenous Alteplase Plus Endovascular Treatment on Functional Independence in Patients with Acute Ischemic Stroke) was recently completed in China. The trial had a prespecified non-inferiority margin of 10% of MT alone versus IVT plus endovascular treatment.58 DEVT indicated that MT alone achieved the non-inferiority to bridging therapy when compared to the 90-day functional independence (MT group 54.3% vs bridging group 46.6%). Three other similar RCTs are in progress (table 2).
Alteplase or tenecteplase with MT
Currently, alteplase and tenecteplase are the main thrombolytic agents to treat patients with AIS. Tenecteplase (TNK), a genetically modified variant of alteplase, is more fibrin specificity, and with a longer half-life, permitting bolus infusion.59 60 In the Extend-IA TNK study, IV-TNK versus IV-Alteplase prior to arteriography for MT was associated with a higher rate of reperfusion >50% (22% vs 10%). The independent functional outcome of patients with AIS-LVO in the TNK group was better than those treated with alteplase within 4.5 hours of symptom onset (median mRS, 2 vs 3, p=0.04).33 A post-hoc analysis of the EXTEND-IA TNK trial concluded that TNK was also more cost-effective.61 The EXTEND-IA TNK Part 2 RCT suggested that for patients with AIS-LVO, intravenous TNK 0.25 mg/kg showed the same benefit as of TNK 0.40 mg/kg before MT.62 TNK might improve the rate of reperfusion for patients with AIS with basilar artery occlusion in comparison with alteplase prior to MT.63 Moreover, improvements in revascularisation and clinical outcomes of intravenous TNK versus intravenous alteplase were confirmed in a meta-analysis.64
First-pass effect and MT devices
First-pass effect (FPE) is defined as achieving complete reperfusion on the first try with the thrombectomy device. An observational study suggested that the FPE was an independent predictor of good clinical outcome, with a 90-day mRS ≤2 in 61.3% in the FPE group versus 35.3% in the non-FPE group.65 Currently stent retrievers and aspiration catheters are the two types of MT devices used to perform thrombectomy.
Seven positive RCTs used stent retrievers as the first-line device for endovascular treatment of AIS-LVO, for example, Solitaire (Medtronic Neurovascular, Irvine, California, USA) and Trevo (Stryker Neurovascular, Fremont, California, USA) stent. However, stent retrievers are continuously improving with new stent material and length, and now are multisegmented with a multifunctional design. The new generation of stents include the EmboTrap II stent (Cerenovus/Johnson & Johnson, New Brunswick, New Jersey, USA), the ERIC stent (MicroVention, Aliso Viejo, California, USA), TIGERTRIEVER stent (Rapid Medical, Yoqneam, Israel), and Versi stent (NeuroVasc Technologies, Laguna Hills, California, USA), and so on.
In 2014, Turk et al reported the initial experience with the direct aspiration first-pass technique (ADAPT) using a large-bore aspiration catheter as the primary method for large vessel recanalisation.10 However, the ASTER study and a 3-Dimensional Stent Retriever with Aspiration-Based Thrombectomy versus Aspiration-Based Thrombectomy Alone study did not suggest aspiration be superior to stent retriever.19 66 In 2019, based on the result of the COMPASS study, the updated AHA/ASA Guideline on treating AIS recommended that direct aspiration as a first-pass MT to be non-inferior to stent retriever for patients with AIS-LVO10 (table 3).
Major clinical trials of adapt
ADAPT technology has certain advantages. First, the time from groin puncture to revascularisation was shorter than those using a stent retriever (25 min vs 33 min, p=0.03). Second, a higher frequent distal aspiration was observed during the stent retriever thrombectomy (85%). Third, the cost of the devices used in the aspiration first-pass group was reduced by an average of US$5074. These findings were observed more in those treated with the larger luminal diameters aspiration catheters Ace 68 (Penumbra Inc, Alameda, California, USA) than the previous ASTER trial Ace 64 ((Penumbra Inc).20 Consequently, in order to improve reperfusion rates in recent years of aspiration catheters, the larger lumen aspiration catheters (diameter between 0.64 and 0.71 inches) came on the market, and initial experience of using the catheters was reported, that is Sofia 6F (MicroVention, Aliso Viejo, California, USA), Catalyst 6 and 7 (Stryker Neurovascular, Fremont, California, USA), JET 7 (Penumbra, Alameda, CA, USA), Millipede 088 (Perfuze, Galway, Ireland) and Zoom 88TM (Imperative Care, Campbell, CA, USA).4 67–70 These reports of initial experience using the new devices indicated the larger bore aspiration catheter may improve suction force and increasing the rate of FPE. However, an issue that deserves more attention is that ADAPT has required rescue stent retriever thrombectomy in about 30% of patients.20
Combining balloon guide catheter (BGC) with stent retriever or/and reperfusion catheter is an effective thrombectomy technique for AIS-LVO. BGCs could temporarily stop the proximal flow and reduce embolic burden, that is treating ICA terminus occlusion, increase one-pass thrombectomy rate, decrease the rate of emboli in new territory and shorten the procedure time.65 71 A systematic review and meta-analysis of five non-randomised studies that included 2022 patients showed that using BGCs during MT had higher odds of first-pass recanalisation and lower mean number of total passes.72 However, a study by the ETIS registry concluded that when combined stent retriever plus contact aspiration are used as a first-line strategy for AIS-LVO, there were no significant difference in achieving a modified thrombolysis in cerebral infarction 2c/3 on the first-pass and clinical outcomes with and without BGC.73
New FlowGate2 (Stryker Neurovascular, Fremont, California, USA), Walrus BGC (Q’Apel Medical, Fremont, California, USA) and Cello (Medtronic Neurovascular, Irvine, California, USA) devices are BGCs with good trackability, greater aspiration power, higher first-pass reperfusion rate and less occurrence of distal emboli.4 74 They will need to be tested for better outcome in clinical practice.
AIS with Intracranial arterial atherosclerosis
Intracranial arterial atherosclerosis (ICAS)-related occlusion is a common cause of AIS, especially in the Asian population.75–77 Hence, the differentiation between an ICAS-related occlusion (ICAS-O) or embolism in patients with a AIS-LVO is a challenge for selecting recanalisation strategies. Yi et al proposed a Microcatheter ‘First-Pass Effect’,”which was to withdraw the microcatheter immediately after it went through the segment of occlusion to allow for blood flow through the occluded site of a LVO. To distinguish ICAS-O from embolism, a series of 61 patients with LVO demonstrated that a positive Microcatheter FPE had a sensitivity of 90.9%, specificity of 87.2% and positive predictive values of 88.5%. At present, it is an accurate method to assess for an ICAS-O with LVO.78 The optional endovascular treatment for an ICAS-O with an AIS-LVO is controversial. Many implies a combination therapy of stent retriever thrombectomy, direct aspiration, glycoprotein IIb/IIIa inhibitor infusion, balloon angioplasty and stenting.77 A previous study of Endovascular Therapy for Acute Ischemic Stroke Trial in China concluded that compared with the embolic group, patients with AIS-LVO with ICAS who underwent standard rescue therapy could achieve a better functional independence at 90 days, but not statistically significant (63.8% vs 51.6%, p=0.169).79 A Systematic Review and Meta-Analysis of the outcome of MT of an ICAS-O showed that the ICAS-O group had a significantly higher rate of intraprocedural intracranial vessel reocclusion than the non-ICAS-O group (36.9% vs 2.7%). Comparing to non-ICAS-O group, the ICAS-O group needed more rescue therapy via balloon angioplasty (9.0% vs 1.3%) and stenting (37.8% vs 2.6%) to achieve a similar rate of recanalisation as in patients with an AIS-LVO. However, there was no statistical difference in the rate of final reperfusion, functional independence, development of sICH and mortality between the two groups.80
Transradial approach for intracranial thrombectomy
A systematic review of 21 studies (n=1342 patients) of using the transradial access (TRA) for neuro intervention, including 46% cases for carotid artery stenting, 32% for aneurysm treatment and 9% for MT.81 Compared with the transfemoral artery access (TFA), TRA may have several advantages: (1) lower risk of peripheral vascular complications (puncture site haemostasis, pseudoaneurysm vagal reflex, etc); (2) lower procedural cost without the need for a vascular closure device for femoral artery puncture sites; (3) shorter recovery time and earlier discharge; (4) less pain at the puncture site; (5) good collaterals for proximal radial artery occlusion during the procedure; and (6) greater patient satisfaction.82 An analysis of 375 TRA cases versus TFA access for MT in the anterior circulation indicated similar median time from image to reperfusion (95 min vs 96.5 min), and 90-day functional independence (mRS 0–2 score) (67% vs 58%). Cross-over from TRA to TFA is higher than TFA to TRA (4.6% vs 1.6%, p=0.088), but without statistical difference. The major complication of the access site is significantly higher in the TFA group than in the TRA group (6.5% vs 0, p=0. 003). It suggests that using TRA for anterior circulation MT may be fast, effective, safe and not inferior to the TFA.83
However, there are several limitations of TRA: (1) it may not use a larger diameter of neurointerventional access catheter or a BGC; (2) difficulty to navigate through sharp turns or anatomy, that is, the acute angle between the carotid artery and subclavian artery in the aortic arch, the occlusion of the subclavian artery and varies types of aortic arch; and (3) require addition training.
Conclusions and future perspectives
At present, MT and intravenous tPA are the most effective reperfusion therapy for patients with AIS-LVO. Patient selection is vital for better functional outcome. Optimal thrombectomy strategy should be developed according to the pathophysiology of an AIS. Studies are ongoing on other issues relevant to the outcome of patients with AIS-LVO undergoing MT such as the use of general anaesthesia or conscious sedation, individualised multimodal neuroimaging, neuroprotection, remote ischaemic preconditioning and artificial intelligence.
Ethics statements
Patient consent for publication
References
Footnotes
Contributors ZM conceived the project. XG wrote the initial draft of the manuscript. XG and ZM revised the manuscript.
Funding This study supported by the National Key R&D Program of China (2016 YFC1301500).
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.