Article Text
Abstract
Background Although stroke management, primary and secondary preventions have been improved in China last decades, the trends and predictors of major vascular events after ischaemic stroke or transient ischaemic attack (TIA) at national scale are less known.
Methods Data were obtained from the three phases of China National Stroke Registry (CNSR), including CNSR-Ⅰ (years 2007–2008), CNSR-Ⅱ (years 2012–2013) and CNSR-III (years 2015–2018). For comparison, patients who were diagnosed as ischaemic stroke or TIA were included. Kaplan-Meier estimates of myocardial infarction (MI) or vascular death were calculated at 1 year. Independent predictors were further assessed with a Cox proportional hazards regression.
Results From 2007 to 2018, a total of 50 284 patients with ischaemic stroke or TIA were enrolled in this study. A declining trend was found in 1-year MI or vascular death (p for trend <0.001), while recurrent stroke depicted a U-shape curve with a nadir in 2012–2013 cohort. A similar trend was also observed in patients who were admitted to 26 hospitals in all three CNSRs. In 2015–2018 cohort, only 251 (1.7%; 95% CI 1.5% to 1.9%) MI or vascular death had occurred at 1 year. Older age, previous stroke or TIA, history of coronary artery disease and the National Institutes of Health Stroke Scale >6 were associated with both an increased risk of MI or vascular death and recurrent stroke. While early antiplatelet therapy and lipid-lowering agents at discharge predicted a reduced risk.
Conclusion A declining trend and current low incidence of MI or vascular death, rather than recurrent stroke, after ischaemic stroke or TIA were observed in China. Traditional factors were found as independent predictors. These findings suggested there is still much room to improve for stroke management.
- stroke
- statistics
Data availability statement
Data are available on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Stroke is the second leading cause of mortality and disability worldwide.1 A previous meta-analysis showed that the risk of myocardial infarction (MI) or non-stroke vascular death after ischaemic stroke or transient ischaemic attack (TIA) was approximately 2% per year2; therefore, stroke was once considered to be a high risk of subsequent cardiac events. In recent years, with improvement of acute management and secondary prevention of stroke, the incidence of MI or vascular death after stroke has declined. For example, a recently conducted meta-analysis indicated that the risk of annual MI followed by ischaemic stroke and TIA was 1.67%, and MI was a less frequent cause of death than recurrent stroke.3 Given the potential heterogeneity among individual studies existed in systematic review, this result should be cautiously interpreted. Furthermore, several multicentre studies conducted in the UK, Japan and South Korea after 20074–6 showed that 1-year incidence of MI after ischaemic stroke decreased to less than 1%, which is more noteworthy.
In China, the annual mortality rate of stroke is approximately 1.6 million of 1.4 billion individuals, exceeding heart disease as the leading cause of adult death and disability.7 Besides, ischaemic stroke account for about 70% of stroke cases, and imposes a heavy burden to the country.8 Previous nationwide surveillance studies have provided reliable estimates for prevalence,9 incidence,9 mortality9 and risk factors10 for ischaemic stroke in China. However, our knowledge is insufficient about the current risk of major vascular events after ischaemic stroke and its aetiology subtypes, in addition to the risk factors, quality of care and secondary preventions related to those factors. Previous two phases of China National Stroke Registry (CNSR) studies have evaluated the quality of care for stoke and reported a substantial progress from 2007 to 2012.11 12 Together with them, the CNSR-III13 provided longitudinal nationwide data to estimate the risk of MI or vascular death after ischaemic stroke or TIA. Therefore, the present study aimed to assess the trend of 1-year risk of MI or vascular death compared with that of recurrent stroke after ischaemic stroke or TIA, report the current 1-year risk of stroke, and investigate independent predictors.
Methods
China National Stroke Registries
The CNSRs are a series of nationalwide hospital-based, multicentre, prospective stroke registries. The primary objective of these registries is to evaluate and improve the quality of care provided during acute hospitalisation. The CNSR-Ⅰ covered 131 hospitals monitored from September 2007 to August 2008, and the CNSR-Ⅱ included 219 hospitals assessed from June 2012 to January 2013. Detailed design, rationale and baseline characteristics of the two registries were described previously.11 12 The CNSR-III recruited consecutive patients at 201 sites from August 2015 to March 2018.13 In order to describe the trend of vascular events, we included the three CNSRs conducted at different time periods, including CNSR-Ⅰ (years 2007–2008), CNSR-Ⅱ (years 2012–2013) and CNSR-III (years 2015–2018). Study population was limited to patients who were diagnosed as acute ischaemic stroke or TIA. Among them, 26 hospitals participated in all three CNSRs, the other hospitals were selected by a steering Committee from the China National Network of Stroke Research including 491 hospitals and matched based on the comparability of the data from the time periods 2007–2008. The flow chart of patient selection was shown in figure 1.
Flow diagram of participants in this study. CNSR, China National Stroke Registry; ICH, intracerebral haemorrhage; IS, ischaemic stroke; SAH, subarachnoid haemorrhage; TIA, transient ischaemic attack.
Outcome evaluation
The primary outcome evaluated in this study was 1-year MI or vascular death. Other composite outcomes, such as MI or non-stoke vascular death and MI or all-cause death were further assessed. Besides, individual MI, recurrent stroke and vascular death were also determined. MI was defined as a rise and fall of cardiac biomarkers (preferably troponin) combined with at least one of the clinical features, such as symptoms of ischaemic strokes lasting >30 min, ECG changes during ischaemia, pathological Q waves, echocardiography and invasive coronary angiography, according to the third universal MI definition. Recurrent stroke was defined as a new ischaemic stroke or haemorrhagic stroke (intracerebral haemorrhage and subarachnoid haemorrhage). All-cause death was classified as vascular or non-vascular. Cause of vascular death included fatal ischaemic stroke, fatal haemorrhagic stroke, sudden cardiac death, fatal MI, fatal heart failure and so on (eg, cardiac arrhythmia, pulmonary embolism, cardiovascular intervention, aortic aneurysm rupture or peripheral arterial disease). Fatal stroke, MI or cardiovascular intervention was defined as an event that was followed by death within 30 days. Non-stroke vascular death excluded the cause of fatal stroke.
Statistical analysis
Continuous variables were expressed as the mean±SD or median (IQR). Categorical variables were presented as number and percentage. Normally distributed continuous variables were analysed using the Student’s t-test or Mann-Whitney U test. For categorical variables, the χ2 method or Fisher’s exact test was used for analysis.
The time trends of all outcomes of CNSRs and OR with 95% CI were calculated. We also tested the time change trend using patients in 26 hospitals as a sensitivity analysis. To calculate cumulative 1-year risk in current 2015–2018 dataset, the first occurrence of MI or vascular death was calculated using the Kaplan-Meier analysis, and cumulative incidence curves were constructed. The risk of selected outcomes was calculated as well. We estimated and compared the risk of the primary outcome in patients categorized according to their age (≤65 vs >65 years old), history of coronary artery disease (CAD, with CAD vs without CAD), final diagnosis (stroke vs TIA) and Trial of Org 10 712 in acute stroke treatment (TOAST) classification (large-artery atherosclerosis vs other types). Comparisons between different subgroups were carried out by the log-rank test. The association of potential predictors with MI or vascular death and recurrent stroke was investigated by Cox proportional hazard model for multivariate regression analyses, and HRs with 95% CI were calculated. Statistical tests were two sided, and p<0.05 was considered statistically significant. All statistical analyses were conducted using SAS V.9.4 software (SAS).
Results
Patients characteristics and outcomes in CNSRs
A total of 50 284 patients with ischaemic stroke or TIA were included in this study, and their clinical characteristics at three study periods are presented in table 1. Patients were numerically younger over time. The prevalence of vascular risk factors in patients during 2015–2018 was similar to the previously reported rate. For in-hospital outcomes, patients in the period of 2015–2018 had a better functional outcome (modified Rankin scale (mRS) ≤2 at discharge) and a lower rate of in-hospital mortality (all p<0.001).
Baseline characteristics and outcomes of three time period cohorts
For 1-year risk of vascular outcomes, the cumulative incidence of MI or vascular death decreased from 11.2% to 7.3% and to 1.7% in the study period of 2007–2018 (p for trend <0.001), which was corresponding to a relative risk reduction of 38% (OR 0.62; 95% CI 0.58 to 0.67) and 87% (OR 0.13; 95% CI 0.12–0.15), respectively (). The declining trend was also found in MI or non-stroke vascular death and MI or all-cause death. However, the risk of recurrent stroke decreased from 2007–2008 to 2012–2013 (17.8% vs 7.3%), and showed a reverse increase from 2012–2013 to 2015–2018 (7.3% vs 9.7%). Besides, the risk of nonfatal MI was 1.3%, 1.4%, and 0.3%, respectively; the risk of vascular death was 10.1%, 6.0%, and 1.5%, respectively. Both parameters shared the same trend (figure 2). A similar trend was also observed in patients who were admitted to 26 hospitals in all three CNSRs (table 2).
One-year outcomes after ischaemic stroke or TIA at different time period (2007–2008, 2012–2013 and 2015–2018). MI, myocardial infarction; TIA, transient ischaemic attack.
Baseline characteristics and outcomes of 26 hospitals included in all three cohorts
Patients characteristics and management between two groups in CNSR-III
Of 15 166 patients in CNSR-III, mean age of patients was 62.2±11.3 years old. The most frequent vascular risk factor was hypertension (62.6%) and the rates of other factors were lower than a quarter. The patients’ baseline data are listed in online online supplemental table S1.
Supplemental material
Patients’ conditions at admission were relatively mild, and the median National Institutes of Health Stroke Scale (NIHSS) and mRS scores were 3 (95% CI1 to 6) and 2 (95% CI 1 to 3), respectively. The vast majority of patients were diagnosed as ischaemic stroke (93.3%). Patients with 1-year risk of MI or vascular death had a higher NIHSS score (>6, 41.4% vs 20.1%), mRS score (>2, 51.4% vs 31.3%), higher rate of large artery atherosclerosis type (35.9% vs 25.3%) and a low rate of Small vessel occlusion type (10.8% vs 21.0%) (online supplemental table S2). For acute management, only 28% of patients were evaluated by a stroke specialist within 24 hours after onset of symptoms. Moreover, rate of intravenous recombinant tissue plasminogen activator (rt-PA) usage was lower than 10%, and lower than 1% for acute endovascular therapy. Median hours from arrival to rt-PA did not significantly differ between the two groups (1.2 hour vs 1.0 hour, p=0.105).
One-year outcomes and predictors in CNSR-III
After 1 year, a total of 251 events of MI or vascular death occurred (222 deaths from vascular events and 39 nonfatal MI cases), which is corresponding to an estimate of 1.7% (95% CI 1.5% to 1.9%). Moreover, MI or non-stroke vascular death was observed in 104 patients (0.7%; 95% CI 0.6% to 0.8%), which was less than 1% (). The Kaplan-Meier estimates of the two composite outcomes during 1 year are shown in online supplemental figure S1.
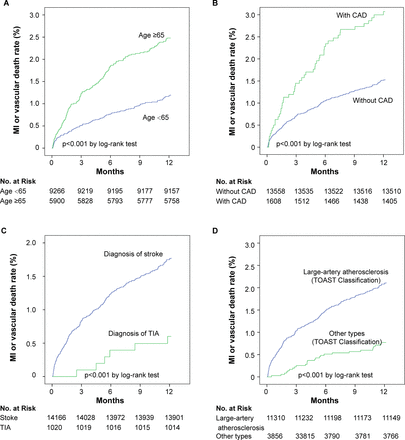
The Kaplan-Meier estimates of MI or vascular death according to the patients’ age, history of CAD, final diagnosis and aetiology of stroke are illustrated in figure 3. Patients with older age, a history of CAD, diagnosis of stroke and large artery atherosclerosis had an increased risk of cardiac events.
Cumulative incidence of 1-year MI or vascular death between different subgroup in 2015–2018 cohort. CAD, coronary artery disease; MI, myocardial infarction; TIA, transient ischaemic attack; TOAST, Trial of Org 10 712 in acute stroke treatment.
In the multivariate regression analysis (table 3), older age, previous stroke or TIA, history of CAD, NIHSS score>6, and diagnosis of stroke were associated with an increased risk of MI or vascular death and recurrent stroke. While early antiplatelet therapy within 48 hours and lipid lowing at discharge decreased the associated risk. Surprisingly, endovascular therapy in acute phase was associated with the adverse outcomes, which may attribute to a low rate of endovascular therapy use. Besides, history of diabetes was associated with an increased risk of recurrent stroke, and history of atrial fibrillation was associated with an increased risk of MI or vascular death.
Predictors of 1-year MI or vascular death in 2015–2018 cohort
Discussion
The current study provided data about 1-year vascular risk in patients who were hospitalised with ischaemic stroke or TIA in China from 2007 to 2018. The main finding is that the trend of MI or vascular death at 1 year significantly decreased in China during three study periods in CNSRs, supporting a significant progress for stroke management, as previously described.12 While the trend of recurrent stroke depicted a U-shape curve with a nadir at 2012–2013, suggesting that there is still a substantial room to improve the quality of care in China. We also briefly reported the patients’ clinical characteristics, management and selected outcomes of patients in CNSR-III. Besides, well-known risk factors for strokes were predictors to the risk. This is one of the largest quality of care studies assessing the temporal trends of vascular events.
There is a gap between adherence to guideline that recommended evidence and clinical practice. Compared with the two successful quality registries, the Get With the Guidelines (GWTG)-Stroke Registry14 and the Sentinel Stroke National Audit Programme,15 the overall quality of adherence to evidence-based recommendation is remarkably lower in China.14 16 Since the central government of the People’s Republic of China launched the first national stroke registry programme to design and evaluate the tools to improve the quality of care in 2007, a persistent progress could be achieved.11 Recently, a cluster randomised clinical trial (Golden Bridge) was conducted to reduce 1-year risk of new vascular events and disability.17 In CNSR-III, the Kaplan-Meier estimate of the risk of the composite outcome for MI or vascular death was 1.66%. Besides, the risk of MI and nonstroke vascular death was 0.69%. The overall incidence of MI or vascular death in the current study was lower than the expected rate (1.7% in our study vs 5.5%–8.2% in the Northern Manhattan study18 in the first year, and was lower than the threshold that was considered to classify patients with high cardiac risk (≥20% at 10 years). Thus, ischaemic stroke and TIA could not be considered as ‘coronary risk equivalents’ at current risk profile and primary prevention therapy. This finding was also consistent with that reported previously.3–6 19
Potential reasons for this declined trend might be related to the lower prevalence of vascular risk factors, the earlier time of presentation (median onset time to arrival, 15 hours), the milder cases (median NIHSS score, 3; median mRS score, 2) in 2015–2018 compared with that in 2007–2008. A decline in mean age at ischaemic stroke onset from 2007 to 2018 might also be a potential reason. In addition, imaging analysis was carried out. Brain imaging, mainly MRI of brain, was conducted in 97.2% of patients, transthoracic cardiac ultrasound in 79.1%, as well as ECG and 24 hours Holter monitoring in 78.7% and 69.7% of patients, respectively. This might contribute to the discovery and treatment of cardiogenic and cryptogenic strokes (data not shown). Therefore, other determined or undermined pathogenies according to the TOAST classification system in CNSR-III accounted for lower than 50%, which is substantially higher than that in other studies.6 20 21 Besides, patients had a satisfactory compliance with secondary preventive measures as evaluated at the time of discharge and at 1 year (data not shown).
Comparably, despite improvement observed in secondary preventive measures, the risk of recurrent stroke did not remarkably vary over time, which was also noted in the recent meta-analysis.3 However, the cumulative incidence of recurrent stroke within 1 year in this study was 9.7%, higher than that in Northern Manhattan (7.7%),22 South Carolina (8.0%),23 South London (7.1%),24 Japan (3.8%),4 South Korea (5.7%)5 and Taiwan (7.8%).25 Consistent with the findings, low rates of acute evaluation by specialist (28%) and intravenous tPA usage (8.6%) were found, both of which are crucial determinants of GWTG-Stroke Registry14 and TIAregistry.org project.20 The barriers might lie in prehospital or in-hospital delay, short treatment time window, and the lack of hospitals’ infrastructure and readiness. Additionally, lower rates of anticoagulants for atrial fibrillation were noted. As international normalised ratio can be monitored in only large tertiary hospitals, several physicians, especially in the low-level hospitals, are therefore reluctant to prescribed warfarin. Also, hypertension is the most important risk factor for stroke in China, highlighting a great opportunity to improve the quality of hypertension management for patients with ischaemic stroke.
The present study showed that the current risk of total MI (fatal and nonfatal) after ischaemic stroke and TIA was below 0.5%, which was significantly lower than that reported before 2015. For instance, a meta-analysis concluded that the annual risk was 2.2% (1.7–2.7) for total MI, 0.9% (0.7–1.2) for non-fatal MI and 1.1% (0.8–1.5) for fatal MI.2 In the Northern Manhattan18 and South Carolina23 studies that recruited stroke patients, the risk of MI was 1.5% and 2.1%, respectively. And in the Rochester Epidemiology Project, the mean annual incidence of MI was 0.95%.21 However, in recent multicentre studies conducted in Japan,4 South Korea5 and the UK,6 the risk of MI at 1 year was between 0.09% and 0.54%, which was much lower. Moreover, in nondisabled patients with TIA and minor stroke in TIAregistry.org project20 and in South Korea,26 the risk of MI was 0.4% and 0.3%, respectively, which was similar to that in CNSR-III. Previous studies confirmed the general assumption that cardiac events remained the main cause of death after ischaemic stroke. Our results were inconsistent with this concept; fatal MI, fatal heart failure and cardiac death accounted for 10.4%, 5.9% and 22.9% of vascular death, respectively. Adding three cardiac causes accounted for less than half of vascular death (39.2%). The result of a recent meta-analysis was also consistent with this inverse trend, and it was reported that the risk of fatal MI was half of the risk of recurrent stroke (incidence rate=0.51).3
Limitations
This study contains several limitations. First, selection of participant sites in CNSRs was associated with selection bias. The sites are more representative of relatively higher medical resources with a better ability of stroke management. Second, of 15 166 patients in 2015–2018 cohort who were analysed, 14 809 (97.6%) had completed 1-year follow-up at the time of this analysis. The lack of 357 patients’ follow-up data might affect the 1-year vascular outcome. Finally, duration of follow-up for MI was not recorded in CNSR-Ⅰ and CNSR-Ⅱ, thus, the time to event analysis could not be calculated in all three CNSRs.
Conclusions
A declining trend and low incidence of MI or vascular death, rather than recurrent stroke, after ischaemic stroke or TIA could be observed in China. Factors, such as age, previous stroke or TIA, history of CAD, NIHSS score and TOAST classification were found as major predictors. Our findings suggested that further research needs to be conducted to improve stroke management.
Data availability statement
Data are available on reasonable request.
Ethics statements
Ethics approval
The study was approved by the Institutional Review Board of the Beijing Tiantan Hospital (IRB approval number: KY2015-001-01).
Acknowledgments
The authors thank the patients who participated in the Third China National Stroke Registry and the contributions of the participating centres.
References
Supplementary material
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors YW and ZJ had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: YW, ZJ and LL. Drafting of the manuscript: LL and ZJ. Critical revision of the manuscript: YP, JJ and CG. Statistical analysis: YP and MW. Study supervision and organisation: YW, ZJ, XM and YJ.
Funding This study was supported by grants from the Ministry of Science and Technology of the People’s Republic of China (2016YFC0901001, 2016YFC0901002, 2017YFC1310901), and grants from Beijing Municipal Commission of Health and Family Planning (No.2016-1-2041, SML20150502), and grants from Beijing Postdoctoral Research Foundation (2020-ZZ-010).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.