Article Text
Abstract
Background Studies show tranexamic acid can reduce the risk of death and early neurological deterioration after intracranial haemorrhage. We aimed to assess whether tranexamic acid reduces haematoma expansion and improves outcome in intracerebral haemorrhage patients susceptible to haemorrhage expansion.
Methods We did a prospective, double-blind, randomised, placebo-controlled trial at 10 stroke centres in China. Acute supratentorial intracerebral haemorrhage patients were eligible if they had indication of haemorrhage expansion on admission imaging (eg, spot sign, black hole sign or blend sign), and were treatable within 8 hours of symptom onset. Patients were randomly assigned (1:1) to receive either tranexamic acid or a matching placebo. The primary outcome was intracerebral haematoma growth (>33% relative or >6 mL absolute) at 24 hours. Clinical outcomes were assessed at 90 days.
Results Of the 171 included patients, 124 (72.5%) were male, and the mean age was 55.9±11.6 years. 89 patients received tranexamic acid and 82 received placebo. The primary outcome did not differ significantly between the groups: 36 (40.4%) patients in the tranexamic acid group and 34 (41.5%) patients in the placebo group had intracranial haemorrhage growth (OR 0.96, 95% CI 0.52 to 1.77, p=0.89). The proportion of death was lower in the tranexamic acid treatment group than placebo group (8.1% vs 10.0%), but there were no significant differences in secondary outcomes including absolute intracranial haemorrhage growth, death and dependency.
Conclusions Among patients susceptible to haemorrhage expansion treated within 8 hours of stroke onset, tranexamic acid did not significantly prevent intracerebral haemorrhage growth. Larger studies are needed to assess safety and efficacy of tranexamic acid in intracerebral haemorrhage patients.
- hemorrhage
- stroke
- CT angiography
- CT
- drug
Data availability statement
Data are available on reasonable request. All data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Spontaneous intracerebral haemorrhage (ICH) is one of the most lethal and disabling form of strokes. Almost 40% of patients with ICH die within the first month and only about 12%–39% of survivors achieve long-term functional independence.1 2 In past decades, many strategies, including blood pressure control, iron supplements and haemostatic therapies have been investigated to treat acute ICH, but few have demonstrated any potential clinical benefit.3
Intracerebral haematoma expansion is a modifiable independent predictor of poor clinical outcome.4 Antifibrinolytic agents such as tranexamic acid may have the potential in reducing intracranial haematoma formation.5 Previous trials investigating recombinant factor VIIa and tranexamic acid for ICH showed a reduction in haematoma expansion, but failed to show a clinical benefit.6 There was even a potential risk of developing ischaemic events.7 8 This lack of clinical effect might be from the unselective inclusion of ICH patients.
ICH patients at a higher risk of haematoma expansion may benefit more from therapies targeting active bleeding. In recent years, several non-contrast CT (NCCT) indicators emerged as promising markers for identification of patients at higher risk of haematoma expansion and unfavourable clinical outcome.9 10 Independent imaging predictors include the blend sign and the black hole sign.11 Furthermore, the spot sign on CT angiography (CTA) is a validated biomarker for active intracerebral bleeding and is associated with clinical deterioration and poor outcome.12–15
We, therefore, designed a randomised controlled trial to investigate whether tranexamic acid could reduce the risk of haematoma expansion in ICH patients with either the spot, black hole or blend sign.
Methods
The protocol and statistical plan of TRAIGE trial has been published earlier.16 Written informed consent was obtained from all patients or their legal representatives before enrolment.
The inclusion process consisted of two periods. Between January 2015 and January 2017, eligible patients aged between 18 and 79 years old presenting to the emergency department at three hospitals in China with an acute primary spontaneous ICH within 6 hours of symptom onset (or time last seen well) and a spot sign were enrolled (online supplemental table 1). A spot sign was defined as a focus of contrast enhancement of a serpiginous or spot-like appearance within a parenchymal haematoma identified on CTA source images without connection to a vessel outside the haematoma margin and corresponding hyperdensity on the NCCT indicative of calcification.17 However, since 2015, two new heterogeneous density on NCCT have been identified as predictors of haematoma expansion in ICH with considerable sensitivity and specificity: the blend and black hole signs.18 19 To better include all ICH patients susceptible to haematoma expansion, broadening the inclusion criteria to include these two signs was proposed. Therefore, between January 2017 and March 2020, an additional seven hospitals recruited eligible primary spontaneous ICH patients with either a blend sign or a black hole sign on NCCT within 6 hours of symptom onset, while the original three hospitals continued to enrol patients with the spot sign throughout the trial. Protocol changes are described in the online supplemental file. Blend sign and black hole sign were of equivalent value in inclusion screening, enrolled patients could be positive of either sign, or both. The blend sign was defined as blending of relatively hypoattenuating area with the adjacent hyperattenuating region within a haematoma with a well-defined margin. The black hole sign was defined as a round, oval or rod-like hypoattenuating area adjacent to the hyperattenuating region within a haematoma with an identifiable border. The spot sign, blend sign and black hole sign were adjudicated in real time by radiologists at each corresponding site not involved in the study. ICH Patients without these signs or declined to participate in the tranexamic acid study were enrolled in an observational registry (online supplemental table 1). All randomised patients received therapy within 8 hours of onset.
Supplemental material
Exclusion criteria included ICH secondary to tumour, trauma, aneurysm, vascular malformation, haemorrhagic conversion of ischaemic stroke, venous sinus thrombosis or central nervous system infection, use of oral anticoagulant therapy with abnormal laboratory values, infratentorial ICH, Glasgow Coma Scale (GCS) score <8, an ICH volume >70 mL, parenchymal haemorrhage expanding to fill one side of the lateral ventricle or more than half of both lateral ventricles, clinical history or current evidence suggestive of venous or arterial thrombotic events within the previous 6 months, pregnancy, within 30 days post partum or lactating, planned surgery for the ICH within 24 hours of onset, contraindication of tranexamic acid and prestroke dependency with a modified Rankin Scale (mRS) score >2. Full inclusion and exclusion criteria is described in the online supplemental file.
Randomisation and masking
Patients were randomly assigned to receive either placebo (0.9% NaCl) or tranexamic acid (1:1) using a computer-generated procedure with randomly permuted blocks of varying size. The treatment number was allocated using a centralised treatment allocation system at the baseline visit. The investigational product was distributed to the participating centres in externally indistinguishable sealed treatment kits containing either tranexamic acid or placebo contained in the identical standard off-the-shelf ampoules. Ampoules and the treatment pack were labelled with a unique pack number. After randomisation, the treatment pack corresponding to the treatment number was handed to a nurse or care provider not involved in patient management or evaluation to be prepared for administration. If there was no occurrence of a serious adverse event due to study medication, the investigators do not have access to the randomisation code throughout the trial. Treatment allocation was concealed from all patients and investigators involved in the trial.
Procedures
All participants received either intravenous tranexamic acid 1 g in 100 mL 0·9% NaCl over 10 min followed by 1 g in 250 mL 0·9% NaCl infusion over 8 hours or placebo, with the same administration regimen. The study drug was to be administered as soon as possible after randomisation, within 8 hours from onset.
Baseline head CT and CTA were assessed for eligibility in real time by enrolling investigators who determined if there was a spot sign, blend sign or black hole sign, and calculated the ICH volumes by using the ABC/2 method.20 Follow-up CT scan was performed at 24 hours (±2) after drug administration to assess for any haemorrhage growth.
Vital signs were closely monitored and recorded during and after the infusion. Investigators were advised to follow the China National Guidelines for the management of spontaneous ICH for blood pressure and general ICH management.21 ECGs were performed at baseline and 24 hours as per protocol. Serum troponin, cerebral imaging, vascular ultrasound, pulmonary arteries CT angiograms, and other auxiliary examinations were conducted as necessary. The published International Health Guidelines (Declaration of Helsinki, 2008) was followed for the handling of data for all ICH patients
Outcomes
The primary outcome measure was the presence of expansion of intracerebral haematoma by 24 hours (±2) after start of drug administration, as defined by an absolute increase of more than 6 mL or a relative growth of more than 33% from the baseline.
Prespecified secondary safety outcomes included major thromboembolic events (acute myocardial ischaemia, acute cerebral ischaemia and acute pulmonary embolism). Safety outcomes were collected through day 90. Secondary efficacy outcome measures included absolute ICH growth volume and absolute intraventricular haemorrhage (IVH) growth volume at 24 hours (±2), poor clinical outcome, defined as death or major disability (mRS 4–6), other thromboembolic events (venous thrombosis and other peripheral arterial embolisms), and death due to any cause, all by 90±7 days.
Statistical analysis
We initially defined a sample size of 240 patients, providing an 80% power to detect a significant absolute difference of 30% in the proportion of patients with haemorrhage enlargement at 24 hours (42% in treatment vs 61% in control arm) at a two-sided statistical significance threshold of p=0·05, and a 10% drop-out rate. Justified by absence of previous efficacy data, we performed a sample size re-estimation with a renewed sample size of 188 participants. Because of the neutral results reported from the STOP-AUST trial, this study was terminated in March 2020, and the final number of patients enrolled was 171.22
The primary analysis was based on the intention-to-treat principle and was conducted according to a prespecified statistical analysis plan. Univariate analyses were performed for examining associations of demographic, clinical, laboratory and imaging variables with clinical outcome. Continuous endpoints were summarised by means or medians, with treatment effects tested by the Student’s t-test or Mann-Whitney U test. Differences between treatment groups are listed as OR and 95% CI. The presence of haematoma growth between the two groups was compared using binary logistic regression. Statistical analysis was done using SAS V.9.4 (SAS Institute).
An independent data and safety monitoring board assessed the safety of the trial participants, and efficacy and overall progress of the study was analysed after all the patients (n=171) were enrolled. The data analysis started after the last patient enrolled completed the 3-month follow-up. Missing outcome values were imputed assuming the worst possible primary outcome (haematoma growth). The primary analysis was unadjusted. The baseline volume was assessed on the most recent scan before randomisation, whether that was the NCCT or CT angiogram. Both baseline and 24 hours ICH volumes were obtained using the ABC/2 method by central independent assessors, who were masked to treatment assignment.
The trial is registered at ClinicalTrials.gov (NCT02625948) entitled ‘Tranexamic Acid for Acute ICH Growth prEdicted by Spot Sign (TRAIGE)’.
Results
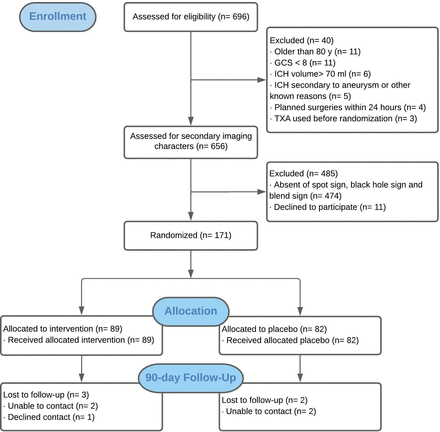
Between 16 January 2015 and 14 February 2020, 696 patients with supratentorial ICH shown on imaging within 6 hours of onset from 10 sites were screened. Among them, 171 (24.6%) were enrolled and underwent randomisation. Of these, 169 (98.9%) patients had 24 hours follow-up CT scans; 89 patients were assigned to the tranexamic acid group; 82 assigned to the placebo group. 2 patients assigned tranexamic acid had missing 24 hours data. All 171 patients received the allocated treatment and were included in the intention-to-treat analysis. By May 2020, a 90-day follow-up for all patients was requested. Day 90 mRS was available in 164 (95.9%) patients (figure 1). Study masking was not broken in any individual. All 171 patients met the inclusion and exclusion criteria.
Trial profile. GCS, glasgow coma scale; ICH, intracerebral haemorrhage; TXA, tranexamic acid.
Their mean age was 55.9±11.6 years, and 124 (72.5%) were male. The median GCS score was 14 (11-15), and the median National Institutes of Health Stroke Scale score was 11 (7–15). The mean volume of ICH at baseline was 23.7±18.7 mL, and median haematoma volume was 19.8 mL (IQR 11.0–31.5). The lobar regions were involved in 44 (25.7%) patients, and the deep grey matter was involved in 127 (74.3%), of which 16 (12.6%) were located in the thalamus and 111 (87.4%) in the basal ganglia. IVH was present in 33 (19.3%) patients. The baseline characteristics of the two treatment groups were similar, except that the platelet count was less in the placebo group, although still within normal range. Furthermore, involvement of the thalamus occurred more frequently in the tranexamic acid group, whereas haemorrhage involving basal ganglia was more frequent in the placebo group (table 1).
Baseline characteristics of the participants
Among the 10 sites, 3 sites enrolled 94 patients used the spot sign on CT angiogram as inclusion criteria, and 7 sites enrolled 77 patients used the black hole sign and/or the blend sign on NCCT as inclusion criteria (online supplemental table 2). More than one sign could be positive in a given patient. Of the 77 patients, 24 (31.2%) had black hole sign and 56 (72.7%) had blend sign. Of 94 patients, 23 (24.5%) had black hole sign, and 51 (54.3%) had blend sign, although these two signs were not regarded sufficient or necessary for inclusion in these three sites (table 1). All 94 patients also had a spot sign.
Median time from onset to arrival at hospital was 120 (69–190) min, onset to treatment was 290 (185–370) min and imaging to treatment was 108 (73–161) min. Eighteen (10.5%) patients were treated within 2 hours after onset, 66 (37.4%) within 4 hours and 123 (70.8%) within 6 hours (table 2 and figure 2). All patients were treated within the 8-hour time window. As shown in table 2, there were no significant differences between the two groups with respect to the medical care provided during the hospitalisation. Pulmonary infection was the most common complication (20 (22.5%) in the tranexamic acid group vs 16 (19.5%) in the placebo group, p=0.64), followed by gastrointestinal bleeding and DVT (table 2).
Time metrics, treatment and complications of the participants
Post hoc forest plot of primary outcome in subgroups stratified by demographic and clinical characteristics. OR less than 1 favours tranexamic acid over placebo. GCS, Glasgow Coma Scale; NIHSS, National Institutes of Health Stroke Scale; SBP: systolic blood pressure; TXA, tranexamic acid.
Haematoma expansion was seen in 70 (40.9%) patients and 58 (34.9%) patients had poor clinical outcome. In the primary analysis evaluating the effect of tranexamic acid on the primary efficacy outcome of ICH expansion, there was no difference between the two groups: 36 (40.4%) of 89 patients in the tranexamic acid group and 34 (41.5%) of 82 patients in the placebo group had haematoma expansion at 24 hours (OR 0.96, 95% CI 0.52 to 1.77, p=0.89). The mean ICH volume change from baseline to 24 hours was 7.1±16.0 mL, being 6.6±16.5 mL in the tranexamic acid group and 7.6±15.6 mL in the placebo group (p=0.70) (table 3).
Primary and secondary outcomes
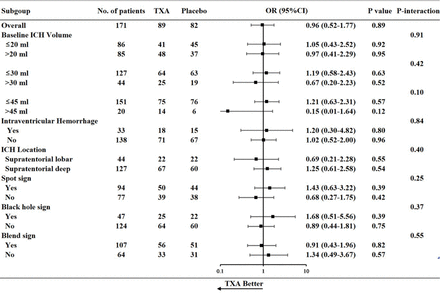
There was no significant difference in the distribution (shift) of the mRS scores at day 90 (GenOR 1.11, 95% CI 0.65 to 1.90, p=0.70) (table 3 and figure 3). The proportion of 90-day mortality from any cause was lower in the tranexamic group than the controlled group (8.1% vs 10.0%, p=0.71). In the prespecified subgroup analysis, there was no heterogeneity of treatment effect by time of administration, whether dichotomised as less than 3 hours vs 3 hours or longer (interaction p=0.85) or as less than 4.5 hours vs 4.5 hours or longer (interaction p=0.14) (figure 4). A trend of benefit of treatment was seen in patients with GCS score greater than 11 (interaction p=0.07) (figure 2). Two patients had major thromboembolic events (acute cerebral infarction), one in each group (p=0.96) (table 3).
Modified Rankin Scale (mRS) distribution at 90 days. A score of 0 represents no symptoms, 1 represents no disability despite symptoms, 2 represents slight disability but able to look after own affairs, 3 represents moderate disability but able to walk without assistance, 4 represents moderately severe disability (unable to walk or attend to own bodily needs), 5 represents severely disabled (bedridden and requiring constant nursing care) and 6 represents death. GenOR=1.11 (0.65 to 1.90), p=0.70. TXA, tranexamic acid.
Post hoc forest plot of primary outcome in subgroups stratified by imaging characteristics. OR less than 1 favours tranexamic acid over placebo. ICH, intracerebral haemorrhage; TXA, tranexamic acid.
Discussion
Our trial was designed on the premise that image-guided patient selection could identify a subgroup of high risk ICH patients that could benefit from tranexamic acid in terms of haematoma expansion. We used three highly predictive imaging biomarkers of haematoma expansion, the spot, blend and black hole signs to select ICH patients for target haemostatic therapy. In patients who had imaging findings suggestive of haematoma expansion and were treated within 8 hours from the onset, tranexamic acid did not result in a significant reduction in haemorrhage growth at 24 hours. This result is consistent with several studies regarding haemostatic therapies in high risk ICH patients.22 23 However, in the secondary outcome analysis, the proportion of death at day 90 was lower among patients assigned to tranexamic acid group compared with the placebo group, although statistically insignificant.
There was a trend of effect of treatment in patients who were treated very early (within 4.5 hours) comparing to those treated later on, though statistically insignificant. The treatment windows used in the subgroup analysis is in accordance to those of (Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage) TICH-2 and (the Spot sign and Tranexamic acid On Preventing ICH growth—AUStralasia Trial) STOP-AUST, both of which also suggested that an earlier treatment window might have a clear effect on reducing haematoma growth.6 22 Despite the effort to do rapid screening, most of the patients in our trial were treated beyond 4.5 hours, which was the reason for the limited power of the subgroup analysis. Furthermore, although tranexamic acid did not reduce haematoma expansion, the proportion of mortality at 90 days was lower in the tranexamic acid group, suggesting potential other mechanisms involved in this positive trending of benefit. Further evaluation testing of tranexamic acid in a tighter treatment window may be warranted.
Baseline haematoma volume is a known strong predictor of haematoma expansion and outcome, particularly in patients with haematoma volume greater than 30 mL.24 In a post-hoc analysis of the TICH-2 trial, participants with a baseline haematoma volume between 30 and 60 mL who received tranexamic acid seemed to have better outcomes.6 In the subgroup analysis, a trend of benefit of treatment was seen in patients with a baseline haematoma volume of greater than 45 mL. It is fair to postulate that patients with moderate size haematoma at baseline might benefit more from tranexamic acid, and could be the targeted population for future studies.
Our study was designed at a time when there were no published results of tranexamic acid treatment on ICH patients; therefore, sample size was indirectly estimated. Based on our results, haematoma growth was observed in 40% of patients, distinctly lower than previously reported, therefore, a positive result could be seen with a substantially large sample size. Recruitment based on a positive spot sign was difficult, as many sites did not routinely perform CTA for ICH patients. Furthermore, to perform CTA probably would require longer time before treatment, as seen in this trial. This appeared as a universal limitation, as two large randomised studies using the spot sign to select patients for haemostatic treatment were terminated prematurely due to recruiting difficulties.23 To mitigate slow enrollment, we expanded our inclusion criteria to include those with newly discovered NCCT findings of blend sign and black hole sign in the second phase of our trial. This trial was the first to screen patients using all three imaging markers. Furthermore, this was the largest randomised controlled trial to test tranexamic acid among patients with acute ICH that were at high risk of haematoma expansion.
The strengths of this study included its double-blinding, allocation concealment, high adherence to treatment protocol and very few missing data on primary outcome. Treatment groups were well balanced at baseline. Some limitations should also be noted. First, it was prematurely stopped with 85% of the planned sample enrolled so the analysis was underpowered. However, as our results highly coincided with other similar trials, our estimates were likely to indicate a true effect. Second, patient outcome may have been confounded by concomitant use of additional agents (blood-lowering therapy, osmotic therapy, statin therapy, etc), which could influence the outcome through unexplained mechanisms. Third, the median delay from arrival at the hospital to treatment of 170 min may have extended the time to treatment, though still within the prespecified time window. As several studies suggested that treating early may be more applicable for haemostatic treatment, this may have limited our findings.
In summary, among patients susceptible to haematoma expansion treated within 8 hours of onset, tranexamic acid did not significantly prevent the growth of ICH. Larger studies with more specified population and very early treatment are needed to further assess safety and efficacy of tranexamic acid in patients with ICH.
Data availability statement
Data are available on reasonable request. All data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author.
Ethics statements
Ethics approval
Ethics approval was reobtained from the Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University in November 2016.
Acknowledgments
Thanks to Fudong Shi, Yaozhi Chen, Xiaoyun Zhang, Xiaochen Wang, Yingying Li, Yanyan Ma and other study coordinators’ meticulous work for data quality control. Thanks to all the participants and investigators who took part in the TRAIGE trial.
References
Supplementary material
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
LL and YW contributed equally.
Collaborators List of Study Sites and Investigators: Sites that recruited patients by CT angiography: Beijing Tiantan Hospital: Liping Liu (PI), Zhonghua Yang, Miao Wen, Ximing Nie, Ying Tan, Yaozhi Chen, Dacheng Liu, Lina Zheng, Jingyi Liu, Jiahui Zhao. Tangshan People’s Hospital: Yan Wang (PI), Mingyang Sun, Wenjian Shi. Tangshan Gongren Hospital: Yibin Cao (PI), Zilong Rao, Yakun Wu, Fengqun Mu, Fengjie Kan, Haiying Wang, Xin Li, Nan Shi, Min Yuan, Yuling Yang, Lingyun Wu, Jingjing Li, Peng Sun, Hong Zhang, Jing Liu, Yueming Tian, Sujie Wang, Qian Li, Lili Chen, Pei Li, Jinghua Liu, Lijuan Liu. Sites that recruited patients by noncontrast CT: Beijing Luhe Hospital: Haomeng Zhu (PI), Huishan Du, Yan’na Tong, Nan Zhang, Fengli Che. Beijing Pinggu Hospital: Yunpeng Zhang (PI), Changbao Li, Yan Wang, Yuming Li, Jincheng Zhang, Jinju Yang Liangxiang. Hospital of Beijing Fangshan District: Lijin Yi (PI), Qingwei Meng, Wenqin Han, Lan Ma, Xinzhang Mu, Jing Yin, Ningning Qin. Kailuan General Hospital: Hebei Ying Ma (PI), Nannan Zhang, Ya Ou, Lifu Zhou, Yujie Sun, Meng Zhao, Lili Zhang, Yesong Liu, Xiaodong Yuan . Beijing Huairou Hospital of University of Chinese Academy of Sciences: Fuying Yu (PI), Lijun Huang, Lixin Song, Jian Wang. Beijing Daxing District People’s Hospital: Fuming Shi (PI), Liping Dong. Beijing Haidian Hospital: Fengchun Yu (PI), Yongzhen Liu, Xiaomei Tang, Wei Liu, Ke Jia, Zhenghong Zhou, Qunyan Li, Hao Feng, Lei Liu, Fenghui Sun.
Contributors JL, XN, HG, QZ, LL and YW had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. YT, DL, LZ, JZ, YW, YC, HZ, YZ, LY, MW, ZY, SS, WW and XZ contributed to the study concept and design. HG, QZ, HS and YP analysed the data. JL, XN, LL and YW drafted the manuscript. All authors have read and approved the final manuscript.
Funding This work was supported by the National Key R&D program of China (2016YFC1307301), National Natural Science Foundation of China (81820108012), National Natural Science Foundation of China (81870913), National Natural Science Foundation of China (81971614) and Beijing Science and Technology Commission (D141100000114002).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.