Article Text
Abstract
Objective Recent years have seen new evidence on the efficacy and safety of dual antiplatelet therapy for secondary stroke prevention. We updated a meta-analysis of randomised controlled trials evaluating dual antiplatelet versus monotherapy for patients with acute non-cardioembolic ischaemic stroke (IS) or transient ischaemic attack (TIA).
Methods We searched PubMed and identified randomised controlled trials evaluating dual antiplatelet versus monotherapy for acute non-cardioembolic IS or TIA within 3 days of ictus up to May 2018. Risk ratio (RR) with 95% CI were calculated using random effects models. Clinical endpoints included stroke recurrence, composite vascular events and major bleeding.
Results 18 randomised controlled trials including 15 515 patients were pooled in the meta-analysis. When compared with monotherapy among patients with acute IS or TIA, dual antiplatelet therapy reduced the risk of stroke recurrence (RR 0.69; 95% CI 0.61 to 0.78; p<0.001) and composite vascular events (RR 0.72; 95% CI 0.64 to 0.80; p<0.001). Dual therapy was associated with a significant increase in the risk of major bleeding (RR 1.77; 95% CI 1.09 to 2.87; p=0.02) when all trial data were combined. However, when all previous trials before the completion of the POINT trial were analysed, dual antiplatelet versus monotherapy was not associated with a significant increase in the risk of major bleeding (RR 1.46; 95% CI 0.77 to 2.75; p=0.25).
Conclusions Among patients with acute non-cardioembolic IS or TIA within 3 days of ictus, dual antiplatelet therapy was associated with a reduction in stroke recurrence, and composite vascular events, when compared with monotherapy. However, a significant increase in the risk of major bleeding was observed.
- antiplatelet therapy
- stroke
- transient ischaemic attack
- meta-analysis
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
Acute minor ischaemic stroke (IS) and transient ischaemic attack (TIA) are very common and often lead to disabling events during the first few weeks.1 Antiplatelet therapy can significantly reduce the risk of vascular events among high-risk patients. Current guidelines strongly recommend early administration of aspirin in patients with acute IS.2 However, the efficacy and safety of dual antiplatelet therapy have not been fully understood.
Over the past few decades, some large randomised controlled trials (RCT) have shown that dual antiplatelet therapy is more effective in reducing the risk of cerebral embolisation, including the CARESS trial (Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis) and CLAIR trial (Clopidogrel plus aspirin vs aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis).3 4 The CHANCE trial (Clopidogrel in High-Risk Patients with Acute Non-Disabling Cerebrovascular Events) further demonstrated that early dual antiplatelet therapy for 21 days among 5170 Chinese patients with non-cardioembolic IS or TIA was efficacious and safe.5 6
A previous meta-analysis including 9012 patients from CHANCE and 13 other RCTs up to November 2012 showed that early dual versus mono antiplatelet therapy within 3 days of symptom onset was more effective in reducing stroke recurrence (risk ratio (RR) 0.69; 95% CI 0.60 to 0.80; p<0.001) and the composite outcome of stroke, TIA, acute coronary syndrome and all death (RR 0.71; 95% CI 0.63 to 0.81; p<0.001), without significantly increasing the risk of major bleeding (RR 1.35; 95% CI 0.70 to 2.59, p=0.37).7 The 2018 American Heart Association/American Stroke Association (AHA/ASA) guidelines recommend the combination of clopidogrel and aspirin among patients with minor IS or TIA for early secondary stroke prevention (Class of recommendation: II A).2 However, considering that the generalisability of the intervention in non-Asian populations remains unclear, the use of dual antiplatelet therapy was only modestly recommended.
Five years after the results of CHANCE were published, POINT (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke), a large double-blind RCT aiming to test the dual antiplatelet therapy with clopidogrel plus aspirin worldwide, has recently published its results.8 9 Therefore, in order to fully explore the efficacy and safety of early dual antiplatelet therapy for secondary stroke prevention, we updated the previous systematic review and meta-analysis of dual versus mono antiplatelet therapy for patients with acute non-cardioembolic IS or TIA within 3 days of symptom onset.7
Methods
Search strategy
We updated the previous systematic review and meta-analysis published in Circulation in 20 137 and 14 eligible studies up to November 2012 were included in this current meta-analysis. We also identified RCTs evaluating dual versus mono antiplatelet therapy for acute non-cardioembolic IS or TIA from November 2012 to May 2018. We searched PubMed and other databases with search words of ‘antiplatelet therapy’, ‘aspirin’, ‘clopidogrel’, ‘cilostazol’, ‘dipyridamole’, ‘ticlopidine’, ‘prasugrel’, ‘triflusal’, ‘glycoprotein IIb/IIIa receptor antagonists’, ‘ticagrelor’, ‘stroke’, ‘cerebral ischemia’, ‘cerebral infarction’, ‘TIA’, ‘transient ischemic attack’, ‘randomized controlled trial’ and ‘randomized trial’. We also performed manual search of references from original articles and pertinent reviews. Searches were restricted to completed trials in human beings and English.
Selection criteria
Two independent authors (YY and MZ) selected all studies. Inclusion criteria for the studies were: (1) RCT in design; (2) dual versus mono antiplatelet therapy was assessed in adult patients (≥18 years) with non-cardioembolic IS or TIA; (3) enrolment and randomisation of all or at least a portion of the patients was within 3 days of the index event; (4) at least one of clinical endpoints was assessed, including stroke recurrence, composite vascular events or major bleeding. Stroke recurrence was mostly defined as ischaemic or haemorrhagic stroke. Composite vascular events were mostly defined as the composite of stroke, TIA, myocardial infarction and death from cardiovascular causes. Major bleeding was mostly defined in accordance with moderate to severe bleeding by the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries definition.10
Data extraction and quality assessment
First, for studies published from November 2012 to May 2018, study quality was independently assessed and data were extracted by two authors (YY and XZ) with the supervision from other authors (YW, XZ, LL). We also repeated data extraction from the 14 studies included in the previous meta-analysis. Unpublished subgroup data from these 14 studies were used directly from the previous meta-analysis. The following data were extracted: publication characteristics, countries or regions of the study, study centres, blinding, enrolled populations, sample size randomised within 3 days of ictus, treatment groups, onset-to-treatment interval, severity of stroke, treatment duration for dual therapy, quality scale, intention-to treat analysis, completeness of follow-up, and efficacy and safety outcomes. The efficacy outcomes evaluated were stroke recurrence and composite vascular events. The safety outcome was major bleeding.
Data synthesis and analysis
Primary analyses were performed for each outcome, with trials subdivided by the different medications assessed. RR and 95% CIs were calculated using random effects models because the interventions, event rates and trial designs were expected to vary. We performed a sensitivity analysis by restricting the analysis to double-blind studies, to test whether the results of the present meta-analysis were sensitive to certain restrictions on the data included. Between-study and between-subgroup heterogeneities were evaluated by calculating the I2 statistic and the Cochrane Q (χ2) statistic. Publication bias of studies with different sample sizes was assessed by performing funnel plots. Two-sided probability values of <0.05 were considered statistically significant. All data were analysed using Cochrane Review Manager (V.5.3).
Results
Selection process and study characteristics
All 14 studies in the previous meta-analysis were included.3 4 6 11–21 For updated relevant studies from November 2012 to May 2018, database searching and citation tracking of references identified 691 publications (online supplementary figure 1). By reviewing title and abstract, 672 articles were excluded. Nineteen articles were reviewed by full text for details, and 15 of them were excluded: not dual versus mono antiplatelet therapy (n=6), no within 3 days of ictus (n=2), not exact onset-to-treatment interval (n=2), not clinical endpoints (n=1) and duplications (n=4). Therefore, four eligible RCTs published after November 2012 were identified, including POINT 2018, COMPRESS 2016, He et al (2015) and Yi et al (2014).9 22–24 All of them compared clopidogrel plus aspirin versus aspirin alone in patients with acute non-cardioembolic IS or TIA.
Supplementary file 1
In total, there were 18 studies with 15 515 patients in the present meta-analysis (table 1), among which 9 were double blind, 11 were intention to treat and 15 had concealed allocation. Six trials enrolled patients with IS only12 15 19 21 22 24, one trial enrolled patients with TIA only11 and the others enrolled both patients with IS and TIA. Seven trials focused on minor stroke.4 6 9 17 18 21 23 Onset-to-treatment intervals were ≤1 day in five trials,6 9 16 18 20 ≤2 days in four trials15 21 22 24 and ≤3 days in the other trials. For those trials that had a recruitment window extending beyond 3 days after the index event, we only used data from those patients recruited and randomised within the 3-day time window.3 13 14 16 17
Design and baseline characteristics of included trials
The following antiplatelet medications were assessed in the meta-analysis: aspirin+clopidogrel versus aspirin (nine trials with 12 404 patients)3 4 6 9 16 18 22–24; aspirin+clopidogrel versus clopidogrel (one trial with 491 patients)14; aspirin+dipyridamole versus aspirin (five trials with 964 patients)12 13 15 17 20; aspirin+dipyridamole versus dipyridamole (two trials with 220 patients)11 13; aspirin+dipyridamole versus clopidogrel (one trial with 1360 patients)19; and cilostazol+aspirin versus aspirin (one trial with 76 patients).21 The European Stroke Prevention Study 2 investigated the combination of aspirin and dipyridamole against aspirin alone and dipyridamole alone, and the other studies each investigated one antiplatelet in the monotherapy group. No studies involving prasugrel, ticlopidine, ticagrelor or triflusal were identified.
Synthesis of results
For analyses of efficacy and safety outcomes, no evidence existed for between-study or between-subgroup heterogeneities by the Cochrane Q statistic and the I2 statistic. No significant publication bias was identified by visual inspection of asymmetry of the funnel plots.
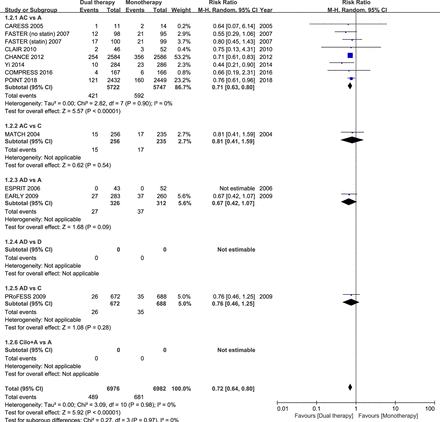
Sixteen studies had data regarding the efficacy outcome of stroke recurrence. In these 16 studies with different follow-up durations, dual antiplatelet therapy reduced the risk of stroke recurrence by ≈30% in patients with acute IS or TIA, as compared with monotherapy (RR 0.69; 95% CI 0.61 to 0.78; p<0.001; figure 1). Eleven studies had data regarding the composite vascular events. Among these 11 studies, dual antiplatelet therapy significantly reduced the risk of the composite vascular events by ≈30% in patients with acute IS or TIA randomised within 3 days of ictus, when compared with monotherapy (RR 0.72; 95% CI 0.64 to 0.80; p<0.001; figure 2).
Comparison of dual antiplatelet versus monotherapy in acute ischaemic stroke or transient ischaemic attack on stroke recurrence. A, aspirin; C, clopidogrel; D, dipyridamole; M-H, Mantel-Haenszel method.
Comparison of dual antiplatelet versus monotherapy in acute ischaemic stroke or transient ischaemic attack on composite outcome of stroke, transient ischaemic attack, acute coronary syndrome and all death. A, aspirin; C, clopidogrel; D, dipyridamole; M-H, Mantel-Haenszel method.
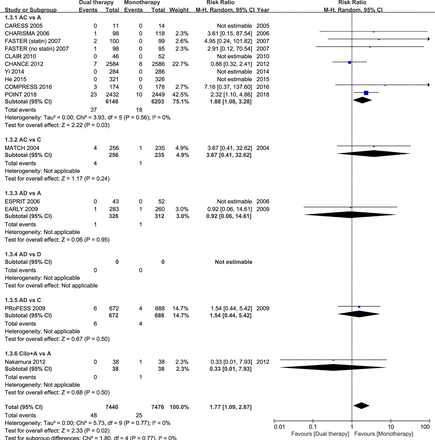
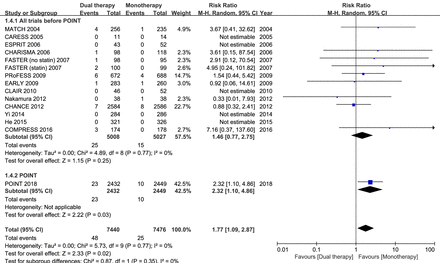
Fourteen studies had data regarding the safety outcome of major bleeding. When all trial data were combined, major bleeding occurred in 0.65% and 0.33% of patients in the dual and monotherapy groups, respectively. As compared with mono antiplatelet therapy, dual therapy for patients with acute IS or TIA was associated with a significant increase in the risk of major bleeding (RR 1.77; 95% CI 1.09 to 2.87; p=0.02; figure 3). However, when all previous trials before the completion of the POINT trial were analysed, dual versus monotherapy was not associated with a significant increase in the risk of major bleeding (RR 1.46; 95% CI 0.77 to 2.75; p=0.25; figure 4).
Comparison of dual antiplatelet versus monotherapy in acute ischaemic stroke or transient ischaemic attack on major bleeding. A, aspirin; C, clopidogrel; D, dipyridamole; M-H, Mantel-Haenszel method.
Comparison of the separate POINT results and the overall estimates of dual antiplatelet versus monotherapy from all other trials included in the present meta-analysis on major bleeding; M-H, Mantel-Haenszel method.
Sensitivity analyses restricted to the nine double-blind trials showed similar results for each outcome (online supplementary table 1) when compared with the full analyses.3 6 9 13 14 16 18 19 22
Supplementary file 2
In the subgroup of the nine RCTs comparing clopidogrel plus aspirin versus aspirin alone, eight trials had dual therapy of ≤3 months (7 days to 3 months). When compared with aspirin alone, the combination of clopidogrel and aspirin was associated with a significant reduction in stroke recurrence (RR 0.69; 95% CI 0.61 to 0.79; p<0.001; figure 1), as well as the composite vascular events (RR 0.71; 95% CI 0.63 to 0.80; p<0.001; figure 2), and there was significant increase in major bleeding (RR 1.88; 95% CI 1.08 to 3.28; p=0.03; figure 3).
The other combinations of dual antiplatelet therapy analysed did not significantly reduce risks of stroke recurrence or the composite vascular events, as compared with monotherapy (figures 1 and 2), though there were no significant between-subgroup heterogeneities throughout the analyses. For trials comparing aspirin plus dipyridamole versus aspirin alone, there were no significant differences between dual antiplatelet therapy and monotherapy on stroke recurrence (RR 0.64; 95% CI 0.37 to 1.10; p=0.11; figure 1), composite vascular events (RR 0.67; 95% CI 0.42 to 1.07; p=0.09; figure 2) and major bleeding (RR 0.92; 95% CI 0.06 to 14.61; p=0.95; figure 3).
Discussion
In this updated systematic review and meta-analysis, 18 RCTs (15 515 patients) evaluating dual versus mono antiplatelet therapy for acute non-cardioembolic IS or TIA within 3 days of ictus were included. We found that, compared with monotherapy, dual antiplatelet therapy was associated with a reduction in stroke recurrence, and composite vascular events, but with a significant increase in the risk of major bleeding. It is likely that good blood pressure control would markedly reduce the risk of intracranial haemorrhage, and diagnosis and treatment of Helicobacter pylori would markedly reduce the risk of major gastrointestinal haemorrhage.
A sensitivity analysis restricted to the nine double-blind RCTs showed similar results, which indicated that results of the present meta-analysis were generalisable. For each outcome, no significant between-study or between-subgroup heterogeneity in treatment effects of dual versus mono antiplatelet therapies was found. The effect of dual antiplatelet therapy on efficacy outcomes in the present meta-analysis was consistent with the results of POINT and CHANCE, while the effect on the safety outcome of major bleeding was not consistent with the overall estimate of all previous trials before POINT.
Both CHANCE and POINT are large randomised, double-blind, placebo controlled, multicentre trials designed to investigate the efficacy and safety for clopidogrel plus aspirin versus aspirin alone in patients with acute minor IS or TIA. However, these two RCTs have some key differences in design. First, the enrolled populations are different. POINT enrolled patients within 12 hours of symptom onset mainly in American and European countries, while CHANCE only enrolled Chinese patients within 24 hours of symptom onset. Second, the antiplatelet therapy adopted in two trials is different. The treatment duration for dual antiplatelet therapy in POINT is 90 days, while the duration in CHANCE is 21 days. Also, the loading dose of clopidogrel in POINT is 600 mg, while the loading dose in CHANCE is 300 mg. Third, the primary efficacy outcome in POINT is a composite of major ischaemic events (IS, myocardial infarction or death from an ischaemic vascular event), while it is stroke (ischaemic or haemorrhagic) in CHANCE. Both trials showed the combination of clopidogrel with aspirin could reduce the risk of stroke recurrence. Therefore, the results of POINT broaden the results of CHANCE to more diverse populations and care setting. However, there was a rate of major haemorrhage of 0.9% in combined antiplatelet group of POINT, significantly higher than 0.4% in the aspirin group of POINT, while the rate of moderate to severe haemorrhage in both groups of CHANCE is 0.3%. It seems that the smaller loading dose of clopidogrel and shorter treatment duration for combined clopidogrel plus aspirin may reduce the risk of haemorrhage. In addition, the frequency of CYP2C19 loss-of-function alleles in Asian population is higher than that in other populations, thus reducing the risk of haemorrhage in CHANCE by poor metabolism of clopidogrel.25 26 These comparisons between POINT and CHANCE further suggest administering short-term dual antiplatelet therapy in the acute phase of IS or TIA is efficacious and safe.27
Another double-blind RCT included in the meta-analysis after November 2012, the COMPRESS trial (Combination of Clopidogrel and Aspirin for Prevention of Recurrence in Acute Atherothrombotic Stroke Study), randomised 358 patients with acute IS caused by large artery atherosclerosis within 48 hours of onset to clopidogrel plus aspirin or to aspirin alone for 30 days.22 However, clopidogrel plus aspirin was not shown to be superior to aspirin alone in reducing new ischaemic lesion recurrence on MRI and clinical vascular events. Only 21.8% of patients were enrolled within 24 hours of onset and a loading dose of clopidogrel was not given, both of which might explain the negative results.
The recently published TARDIS trial (Triple Antiplatelets for Reducing Dependency after Ischaemic Stroke), an international, open-label, blinded-endpoint, superiority RCT,28 compared the safety and efficacy of intensive (combined aspirin, clopidogrel and dipyridamole) versus guideline-based (either clopidogrel alone or combined aspirin and dipyridamole) antiplatelet therapy in 3096 patients with acute non-cardioembolic IS or TIA within 48 hours of onset. The TARDIS trial was not included in the present meta-analysis, because it focused on triple versus mono or dual antiplatelet therapy. In TARDIS, triple antiplatelet therapy did not reduce the incidence and severity of recurrent stroke or TIA, but did significantly increase the risk of major bleeding, suggesting triple antiplatelet therapy should not be used.29
The SOCRATES trial (Acute Stroke or Transient Ischemic Attack Treated with Aspirin or Ticagrelor and Patient Outcomes) compared ticagrelor with aspirin in an international population, so it was not included in our meta-analysis.30 The main SOCRATES analysis found that ticagrelor was not superior to aspirin in reducing the risk of major vascular events.31 However, ticagrelor was superior to aspirin in large artery disease,32 and there was a trend to superiority in Asian patients.33 Two other relevant RCTs are ongoing. The international THALES trial (Acute Stroke or Transient Ischemic Attack Treated with Ticagrelor and ASA (acetylsalicylic acid) for Prevention of Stroke and Death) aimed to demonstrate the superior efficacy of ticagrelor plus aspirin versus aspirin alone in the prevention of subsequent stroke at 30 days in patients with IS or TIA (ClinicalTrials.gov number: NCT03354429). THALES will be covered in an updated meta-analysis like this one. In addition, the CSPS.com trial (Cilostazol Stroke Prevention Study for Antiplatelet Combination), a multicentre, open-label RCT, is evaluating the efficacy and safety of dual antiplatelet therapy involving cilostazol for secondary stroke prevention.34 A total of 4000 high-risk patients with non-cardioembolic IS will be randomised 8–180 days after onset to dual therapy with cilostazol plus aspirin or clopidogrel, or to aspirin or clopidogrel monotherapy for at least 1 year (ClinicalTrials.gov identifier: NCT01995370). However, CSPS.com excluded patients within 3 days of ictus.
There are several limitations of the meta-analysis. First, included studies varied in characteristics, including the study population, stroke severity, antiplatelet medications, onset-to-treatment interval, treatment and follow-up durations, and other aspects. All of these factors could be potential confounders. Second, in some included studies, patients with IS or TIA within 3 days of ictus were not the primary target population and were a small portion of the primary study populations. Baseline characteristics might not be well balanced between dual and monotherapy groups in these studies.
Conclusions
Among patients with acute non-cardioembolic IS or TIA within 3 days of ictus, dual antiplatelet therapy was associated with a reduction in stroke recurrence, and composite vascular events, when compared with monotherapy. However, a significant increase in the risk of major bleeding was observed, which might attribute to higher loading dose of clopidogrel and longer treatment duration for dual therapy. The current data suggest administering short-term dual antiplatelet therapy in the acute phase of IS or TIA is efficacious and safe.
References
Footnotes
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent Not required.
Provenance and peer review Commissioned; internally peer reviewed.
Data sharing statement Additional data that supports the finding of this study is available from the online supplement.
Guest chief editor J David Spence