Article Text
Abstract
Introduction Cerebral ischaemia-induced depression is among the most frequent neuropsychiatric consequences and adversely impact the prognosis and recovery of patients. Although several brain regions have been implied in the development of ischaemia-induced depression, the brain region-specific neural cell apoptosis pathways have not been clarified yet.
Methods In this study, bilateral internal carotid artery occlusion (BICAO) mouse model was established to induce cerebral ischaemia. Sucrose preference, tail suspension and forced swim tests were conducted on mice at 7, 21 and 30 days after BICAO treatment. In addition, brain regional ischaemic neuron loss was investigated by using immunofluorescent staining of neuronal nuclei (NeuN) and caspase-8/-9-dependent cell apoptosis was also examined by western blot analysis.
Results BICAO-induced cerebral ischaemia resulted in decreased sucrose preference and increased immobility times, which were representative depressive-like behaviours of mice until 30 days after BICAO treatment compared with Sham-operated mice. This outcome was associated with significant neuron loss by using immunofluorescent staining and increased cleavage levels of pro-caspase-3/-8/-9, but not pro-caspase-12, by western blot analysis in hypothalamus, midbrain, prefrontal cortex and hippocampus of mice.
Conclusions This study showed that BICAO-induced ischaemia caused depressive-like behaviours and caspase-8/-9-dependent neural cell apoptosis in several brain regions, including hypothalamus and midbrain of mice.
- depression
- apoptosis
- bilateral internal carotid artery occlusion
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
Up to now, approximately one-third of stroke victims are estimated to be clinically depressed.1 2 Characterised by negative mood, diminished interest or pleasure (anhedonia), low self-esteem, lack of motive and social withdrawal, cerebral ischaemia-induced depression leads to a higher risk of subsequent stroke, poor recovery and increased healthcare costs.3–5 Moreover, only less than 50% of patients with major depressive disorder under current antidepressant treatments result in remission and it remains unknown whether patients with depression with cerebral ischaemia respond better to specific kinds of antidepressant medicine.6–8
During the last decade, limited progress has been made in the understanding of biological mechanism and development of effective pharmacological therapies due to limited appropriate animal models. Although complicated and under debate, increased neural cell apoptosis, along with dysregulation of the hypothalamus–pituitary–adrenal axis and impaired neurogenesis, has been implied in the aetiology of depression.9–14 Recently, APAF-1, encoding the protein apoptosis protease activating factor 1, was identified as the major predisposition gene in high-risk major depressive disorder pedigrees. Since Apaf-1 could activate caspase-9 and thus initiate intrinsic apoptotic pathway, these findings for the first time implied that aberrant increase of apoptosis may play an important causative role in, rather than a symptom of, major depressive disorder in a subgroup of patients carrying specific alleles of APAF-1.15 Meanwhile, an elevation in Bax/bcl-2 ratio and TUNEL-positive neurons were also demonstrated in focal ischaemia-induced depressive rat model.16 Moreover, anti-apoptotic effects of antidepressants such as tianeptine have been determined in several clinical and experimental studies.17–19 However, few studies have discussed the role of apoptosis in specific brain regions other than cortex and hippocampus. In order to gain a better understanding of the association between brain regional apoptosis pathways and depression, extensive in vivo evidence is badly needed.
Bilateral internal carotid artery occlusion (BICAO) is a rare but important cause of ischaemic stroke.20 As a result of severe reduction in blood perfusion and energy disruption, BICAO caused extensive cerebral ischaemic injuries.21 Although brain regions such as cortex have been implied in the development of ischaemia-induced depression, the brain region-specific neural cell apoptosis pathways have not been clarified yet. In this study, we aimed to investigate which brain regions were mostly affected and associated with post-stroke depression and the underlying cell apoptosis pathway. Using BICAO mouse model, the neuropsychiatric behavioural outcome of C57BL/6J mice was examined and the brain regional neuron loss and cell apoptosis levels were further investigated.
Methods
Most chemicals were obtained from Sigma-Aldrich (St. Louis, Missouri, USA), while some specific agents were purchased from the indicated companies in the text. Male C57BL/6J mice, at the age of 8–12 weeks old (Jackson Laboratory, Bar Harbor, Maine, USA) and weighing 20–28 g, were used in this study. The mice were housed in a 12/12-hour light/dark cycle with free access to food and water. A total of 32 mice were used (figure 1). For behavioural tests and immunofluorescent staining, the mice were randomly divided into either Sham (n=10) or BICAO (n=10) group. For western blot analysis, 12 additional mice were used, including six in Sham group and six in BICAO group.
Experimental timeline. Thirty-two mice were randomly divided into BICAO and Sham operation groups. The behavioural tests of sucrose preference, tail suspension, forced swim and pole tests started at days 7, 21 and 30 after Sham or BICAO treatment (n=10 per group). After the last behavioural experiment, 20 mice (n=10 per group) were sacrificed and mouse brains were processed for immunofluorescent staining of neuronal marker neuronal nuclei (NeuN). For western blot experiments, 12 mice (n=6 per group) were sacrificed 1 day after Sham or BICAO treatment. BICAO, bilateral internal carotid artery occlusion; FST: forced swim test; IF, immunofluorescence; PT: pole test; SPT, sucrose preference test; TST, tail suspension test.
BICAO mouse model
The BICAO was used to induce global ischaemia of mice. This model was modified according to a previous report.22 Briefly, under 1% pentobarbital sodium-induced anaesthesia, bilateral carotid arteries were exposed followed by a ventral midline neck incision. The internal carotid arteries were dissected at the bifurcation and ligated permanently using a 6–0 surgical suture. Sham-operated mice underwent the same procedures of anaesthesia, incisions and internal carotid artery dissection without the ligation of bilateral internal carotid arteries. Subcutaneous infiltration of long-term lidocaine after suture was applied as analgesic to both groups of mice. During this surgical treatment, mouse rectal temperature was maintained at 37°C with the use of a heating lamp and thermal blanket. Afterwards, mice were kept warm and undisturbed in a temperature and humidity-controlled animal intensive unit for a minimum of 2 hours. In the following recovery days, the intake of food and water and behavioural signs of post-surgery pain (if any) were also monitored.
Immunofluorescent staining
Mice with 30-day BICAO treatment were sacrificed for immunofluorescent staining. According to our previous report,23 mouse brains were transcardially perfused with phosphate-buffered saline (PBS, pH 7.4) followed by paraformaldehyde and dehydrated for 24 hours. respectively. in a cold room. Then, the cryo-protected mouse brains (frozen in liquid nitrogen for 4 min) were serially sectioned into 20 µm thickness coronal slices using a cryostat microtome (CM1950 Clinical Cryostat, Leica Biosystems, Germany). According to the Bregma, the following brain regions were stereotaxically positioned: prefrontal cortex (+3.08 to +2.58 mm), paraventricular nucleus (PVN) of hypothalamus and dorsal hippocampus (−0.94 to −2.30 mm) and substantia nigra core (SNc) subregion of ventral midbrain (−3.08 to −3.80 mm). Five slices that were equally spaced through the longitudinal axis of each mouse brain region were selected. The procedures of immunofluorescent staining were as follows. First, 8% goat serum dissolved in 0.5% Triton X-100 in 0.1 M PBS was used for 1 hour to block the background noise signal. Afterwards, brain slices were incubated with neuron-specific marker (neuronal nuclei (NeuN), 1:500, ab104224, Abcam, Boston, Massachusetts, USA) at 4°C overnight and with secondary antibody for 2 hours at room temperature after rinsing. The second antibodies were Alexa Fluor 594 Goat anti-mouse IgG (1:250, A11029, Molecular Probes, Thermo Fisher Scientific, Rockford, Illinois, USA). Followed by 3 times thorough rinsing after primary and secondary antibodies incubation with PBST solution, we used 2-(4-amidinophenyl)−6-indolecarbamidine dihydrochloride containing mounting media to mount the slices in dark room. Four images were taken randomly (two at one side) within interested region of every slice using Leica microscope (image size: 324.9 µm×243.1 µm). By using Image J software, neuron loss was counted as follows: the percentage of (Sham-operated neurons–BICAO-treated neurons) in total Sham neurons.
Evaluation of behaviours
The sucrose preference, forced swim test, tail suspension and pole test were applied on 7, 21 and 30 days following Sham or BICAO treatment as the scheme, as indicated in figure 1. For all behavioural evaluations, mice were habituated to the testing room for at least 30 min before being tested. Sucrose preference test was used to evaluate interest of mice in seeking a sweet rewarding drink relative to plain water.24 Before the test, 1% sucrose solution or distilled water was freshly prepared and each mouse was separated in a single cage for at least 30 min. During test, two identical plastic bottles containing different solutions, respectively, were weighted and carefully inserted into each cage at a random location. Mice were allowed free access to both bottles and food without disturbance for 24 hours. After the test, bottles were removed and the reduced weight of each bottle was recorded and represented the consumption of each solution. The decreased sucrose preference levels, which were calculated as the percentage of 1% sucrose solution consumption to total consumption of two bottles, indicated anhedonia-like symptoms in human beings and rodents.25 Forced swim and tail suspension are common tests to measure ‘learned helplessness’ behaviours of rodents in an environment where they cannot escape from. In forced swim test, each mouse was individually placed in a transparent plexiglas cylinder (30 cm in height; 15 cm in diameter) containing water (15 cm in water height, temperature maintained between 21oC and 23oC). The whole process lasting 6 min was videotaped. According to previous reports,26 27 the time when the mice was not trying to swim or escape but only floating, with or without making subtle movements to keep its head above the water, was defined as the immobility time. After the test, the mice were withdrawn from the water, dried and returned to their cages. Immobility time during the last 4 min was measured. Tail suspension, compared with forced swim test, is only recommended in mice.28 Each mouse was suspended individually by its tail top using pieces of adhesive tape and behaviours were videotaped. In order to prevent tail climbing movement, smooth plastic cylinders (1 cm in diameter and 1.5 g in weight) were placed prior to the test and rested at the base of each mouse tail after they were suspended. Each animal was tested only once and out of view from the other animals. The mice were suspended from a flat metal bar 30 cm above the table for 6 min. After the test, the mice were returned to their cages and the device was cleaned with 70% ethanol solution. In our test, escape-related behaviours included strong shake of the body and movements of the limbs akin to running. Pendulum-like movements and subtle movements of forelimbs without hindlimbs were not regarded as escape-related behaviours. After the test, the time of mice without typical escape-related behaviours during the last 4 min was measured. The pole test was used to test the general motor function of mice using a rough-surfaced vertical placed pole (5 cm in diameter; 80 cm in height) as previously described.29 30 First of all, the mouse was placed head upward on the top of the pole, then it was let go and the time until it descended to the floor with its hindlimbs was recorded and compared between groups.
Western blot analysis
Twenty-four hours Sham and BICAO-treated mice were sacrificed for western blot analysis. Specific mouse regions were dissected, fast frozen in liquid nitrogen and homogenised and sonicated in Buffer C (detailed composition of Buffer C was reported previously31). Afterwards, protein concentration was determined via BCA Kit (Pierce Company, Rockford, Illinois, USA) and the total protein of 60 µg in each sample was loaded on 12% sodium dodecyl sulfate polyacrylamide gel. Polyvinylidene difluoride membrane was used to transfer the proteins in gel onto the membrane (0.22 µm, GE Healthcare, Little Chalfont, UK) and later blocked with 10% non-fat milk dissolved in Tween-20/Tris-buffered system for 1 hour with gentle shaking. Incubation with primary antibodies lasted overnight in a 4° cold room, followed by 1 hour incubation of horseradish peroxidase-conjugated secondary anti-rabbit (Stressgen Biotechnologies Corp., Victoria, British Columbia, Canada) and anti-mouse antibodies (Jackson ImmunoResearch Labs Inc., Pennsylvania, USA). The target protein primary antibodies in this study included caspase-3 (anti-rabbit polyclone,1:500; #9665s, CST, Danver, Massachusetts, USA), caspase-8 (anti-mouse monoclone,1:500; #C4106, Sigma-Aldrich), caspase-9 (anti-mouse monoclone,1:500; #C4356, Sigma-Aldrich) and caspase-12 (anti-rat monoclone, 1:500; #C7611, Sigma-Aldrich). The band signals were detected using enhanced chemiluminescence Kit (GE Healthcare) and Gel Doc 2000 Imaging System, while density quantification was performed by using analysis tools of Quantity-One software (Bio-Rad, Hercules, California, USA). All the data were statistically normalised to the endogenous loading control β-actin (1:10000, #60 008–1-Ig, Proteintech Corp., Illinois, USA).
Statistical analysis
The data in this study were presented as mean±SE of the mean. One-way analysis of variance and Bonferroni were performed for all pairwise multiple comparisons (GraphPad Prism V.6.00, La Jolla, California, USA). The P value of <0.05 was considered as statistically significant.
Results
BICAO-induced cerebral ischaemia elicited depressive-like behaviours of mice
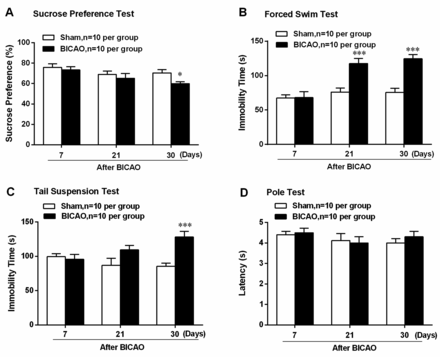
To determine whether BICAO-induced cerebral ischaemia elicited depression-like behaviour or altered motor function, multiple behavioural tests were adopted on both Sham-operated and BICAO-operated mice. Beginning at 7 days after global ischaemia, several behavioural tests were conducted sequentially, with the least stressful sucrose preference test first, then the pole test, tail suspension and forced swim test, as was shown in the scheme in figure 1. Sucrose preference test results showed significant decreased preference for sucrose solution in mice with BICAO treatment compared with Sham-operated mice at 30 days after surgery (F (5,54)=2.643, P=0.0172, n=10 per group, figure 2A). In addition, the immobility time of mice with BICAO treatment was significantly longer in forced swim test at 21 and 30 days (F (5,54)=8.654, P<0.001, n=10 per group, figure 2B) and in tail suspension test at 30 days (F (5,54)=5.341, P<0.001, n=10 per group, figure 2C) after surgery compared with mice with Sham treatment. Next, we also assessed whether BICAO-induced global ischaemia could affect motor function of mice. The results of pole test showed that the latency to run through the pole was not significantly different among Sham and BICAO-treated mice at 7, 21 or 30 days post-surgery, suggesting the motor function of mice with global ischaemia was not impaired significantly (F (5,54)=0.6750, P>0.05, n=10 per group, figure 2D). Collectively, these results suggested that BICAO-induced global ischaemia resulted in significant depressive-like behaviours of mice in the subacute stage.
Bilateral internal carotid artery occlusion (BICAO)-induced cerebral ischaemia elicited depressive-like behaviours of mice. (A) The result of sucrose preference test showed that mice with 30 days BICAO treatment exhibited decreased sucrose solution preference compared with Sham group (n=10 per group). *P<0.05. (B) The results of forced swim test showed that mice with 21 and 30 days BICAO treatment had significant longer immobility time compared with Sham group (n=10 per group). ***P<0.001. (C) In tail suspension test, prolonged immobility time was also observed in mice with 30 days BICAO treatment compared with Sham group (n=10 per group). ***P<0.001. (D) Meanwhile, there was no significant difference in the latency of mice to descend the pole between Sham and BICAO groups at 7, 21 and 30 days after ischaemia (n=10 per group).
BICAO induced significant neuron loss in emotion regulation brain regions of mice
As previously reported, BICAO induced significant decrease of blood perfusion in several brain regions. Thus, we aimed to examine which brain regions were associated with depressive-like behaviours post ischaemia. By using immunofluorescent staining of NeuN, we focused on neuronal loss in brain regions which are particularly involved in emotional, cognitive and reward processing functions. Typical staining images (figure 3A) and quantitative analysis results demonstrated significant neurons reduction in the paraventricular nucleus (PVN) of hypothalamus, Substantial Nigra core (SNc)of midbrain, layer IV of prefrontal cortex, the cornus ammonis (CA) 1 and 3 of hippocampus of 30-day BICAO-treated mice compared with Sham-operated mice. In addition, the neuron loss percentages of hypothalamus and midbrain were significantly higher than those of prefrontal cortex and CA1 and CA3 of hippocampus (F(4, 45)=24.77, P<0.001, n=10 per group, figure 3B). These data suggested that BICAO resulted in significant neuron loss in the hypothalamus, midbrain, prefrontal cortex and hippocampus. Of note, hypothalamus and midbrain were more vulnerable to BICAO-induced ischaemia.
Bilateral internal carotid artery occlusion (BICAO) induced significant neuron loss in emotion regulation brain regions of mice. (A) The typical staining images of neuronal marker neuronal nuclei (NeuN) demonstrated significantly reduced NeuN-positive neurons in the paraventricular nucleus (PVN) of hypothalamus, substantia nigra core (SNc) of midbrain, prefrontal cortex and the cornus ammonis (CA) 1 and 3 of hippocampus of 30-day BICAO-treated mice compared with Sham group (scale bar=50 µm). (B) The quantitative analysis results showed that neuronal loss percentages of hypothalamus and midbrain were significantly higher than those of other regions (n=6 per group). ***P<0.001 versus neuronal loss percentage in other regions of mice.
Caspase-8/-9-dependent cell apoptosis was involved in emotion regulation brain regions of mice
As an executioner, caspase-3 can be activated in the apoptotic cell by extrinsic (caspase-8 mediated), intrinsic (caspase-9 mediated) and endoplasmic reticulum stress-associated (caspase-12 mediated) pathways. However, which apoptotic pathway participated in BICAO-induced global ischaemia has not been well established. To this end, the western blot analysis was adopted to detect the region-specific cell apoptosis levels. The cleavage levels of pro-caspase-3, -8, -9 and -12 were determined in above-mentioned regions of mice after 24 hour BICAO treatments. The typical western blot images (figure 4A) as well as quantitative analysis results showed that the cleavage levels of caspase-3 (F(4,25)=93.72, P<0.001; n=6 per group, figure 4B), caspase-8 (F(4,25)=5.455, P<0.01; n=6 per group, figure 4C) and caspase-9 (F(4,25)=5.045, P<0.01; n=6 per group, figure 4D) but not caspase-12 were significantly higher in hypothalamus, midbrain, prefrontal cortex and hippocampus compared with Sham group. These results suggested that endogenous caspase-8 and -9-dependent cell apoptosis pathways participated in BICAO-induced global ischaemia and may subserve a pathophysiological role in the associated depressive-like behaviours of mice.
Caspase-8/-9-dependent cell apoptosis was involved in emotion-related brain regions of mice. The typical western blot images (A) and quantitative analysis results demonstrated increased cleavage levels of caspase-3, -8 and -9 but not caspase-12 in hypothalamus, midbrain, prefrontal cortex and hippocampus of mice with 24 hours bilateral internal carotid artery occlusion (BICAO) treatment compared with Sham group ((B), (C) and (D), n=6 per group). ***P<0.001 compared with Sham group.
Discussion
Using a battery of neurobehavioural tests, this study showed that BICAO-induced ischaemia caused apparent depressive-like behaviours of mice with 30-day BICAO-induced global ischaemia, and these changes were associated with significant neuron loss and increased caspase-8/-9-dependent cell apoptosis pathways in emotion regulation brain regions, including hypothalamus, midbrain, prefrontal cortex and hippocampus.
Although many studies on depression have been conducted, the brain regional pathophysiological mechanisms underlying ischaemia-induced depressive disorders still remain to be elucidated. To our knowledge, this is the first study exploring the effect of permanent global ischaemia on neuropsychiatric behavioural changes in subacute stage using BICAO mouse model. This model has better clinical relevance to the internal carotid artery stenosis or occlusion. The quick motor function recovery of mice with BICAO-induced global ischaemia, which was proved in the pole test at 7 days post-surgery, makes it more suitable for behavioural studies compared with other global ischaemia models. In addition, unlike 4-vessel occlusion (4VO) and cardiac arrest models, the BICAO model has a nearly 100% survival rate. While the cerebral regional perfusion was significantly decreased, the blood flow of the external carotid arteries and vertebral arteries was preserved, which is fundamental to the normal function of the thyroid and facial and masticatory muscles.
The natural course of depression after ischaemia remains an issue under debate. De la Tremblaye and colleagues determined that rats with 4VO-induced global ischaemia displayed characteristics of depression at 7 and 14 days using sucrose preference and forced swim tests.32 In the present study, prolonged immobility time was first observed in mice at 21 days after BICAO using forced swim test. At 30 days after ischaemia, this characteristic was again determined by both tail suspension and forced swim test. Meanwhile, significant decrease in preference for sucrose was also examined at the time point of 30 days post ischaemia. So in this case, the BICAO-induced depressive-like behaviours were valid in mice with 30 days BICAO treatment. The divergent findings on occurrence of depressive-like behaviours may be explained by the different ischaemia paradigms, including focal ischaemia. Understanding the different occurrences of cerebral-induced depression is of vital clinical importance to the treatment strategy.
In the 1970s, the identification of depression following a stroke led to the concept that post-stroke depression could be a consequence of brain damage.33 34 Supported by many clinical and animal studies, disruption of neural circuits/chemicals involved in several emotion regulation regions was the main biological aetiology.35 36 However, which brain region was involved and its sensitivity to ischaemia still remain to be explored. De la Tremblaye et al found reduced brain-derived nerve growth factor and TrkB level in the mPFC and heightened levels in the NAc 30 days post 4VO-induced global ischaemia.32Hippocampal neuron loss (CA1 and CA3 subregions) was also observed at 8 and 16 days post global ischaemia.37 38 It was noteworthy that the hypothalamus and midbrain did not earn much research focus on its vulnerability to ischaemia. In this study, we discovered significant neuron loss in hypothalamus, midbrain, prefrontal cortex and hippocampus of mice with 30 days BICAO-induced global ischaemic injury using immunofluorescent staining of mature neuron marker NeuN. Interestingly, significantly higher neuron loss percentages of hypothalamus and midbrain indicated that these two regions suffered much more than other regions. Therefore, hypothalamus and midbrain being more sensitive to the deleterious effect of global ischaemia may to some extent contribute to fast dysregulation of hypoxic–pituitary–adrenalin axis and hence the induction of depression.
Cell apoptosis is a normal physiological programmed cell death that can be enhanced by a variety of external stimuli, including ischaemic injury. Using TUNEL assay, low-level apoptosis was detected in entorhinal cortex, subiculum, dentate gyrus, CA1 and CA4 subregions of hippocampus in 11 of 15 depressed patients and 1 of 16 control subjects.39 Wang and colleagues observed increased TUNEL-positive neurons in hippocampal dentate gyrus of rats with chronic mild stress accompanied by MCAO-induced ischaemia.16 Given the fact that TUNEL assay was only a method to reflect ongoing apoptosis at the time of death, these findings did not give an accurate clue about the relationship between apoptosis and depression. Yet, assessments of neural cell apoptosis in the brain after ischaemia have been focused mainly on hippocampus and cortex and very little is known about the cell apoptosis level in other brain regions. In the present study, we intended to explore the possible underlying cell apoptosis pathways in several less focused brain regions. As an executioner, caspase-3 can be activated in the apoptotic cell by extrinsic (caspase-8 mediated), intrinsic (caspase-9 mediated) and endoplasmic reticulum stress-associated (caspase-12 mediated) pathways. So, in this study, the results that increasing cleavage levels of caspase-3/-8/-9 but not caspase-12 are observed in hypothalamus, midbrain, prefrontal cortex and hippocampus indicated that both caspase-8/-9 involving intrinsic and extrinsic cell apoptosis might contribute to BICAO-induced depressive-like behaviours. Further experiments are needed to determine the direct causal relationship and the detailed mechanisms.
In conclusion, our findings indicated that BICAO-induced ischaemia caused depressive-like behaviours, including anhedonia and learned despair in the subacute stage and brain regional caspase-8/-9-dependent neural cell apoptosis in mice. The study introduced a novel mouse model relevant to ischaemia-induced depression and meanwhile shed new light on the significant role of brain regional apoptosis in the ischaemia-induced depression and thus may give rise to novel treatment strategies.
References
Footnotes
Contributors All the listed authors have participated actively in the study and approved the submitted manuscript. SL and JL: responsible for integrity of the entire study, study concepts and approved the final version of the manuscript to be published. SH and QD: data acquisition and analysis. SL: conducted the experimental studies and drafted the manuscript.
Funding This work was supported by grants from the Seed Grant of International Alliance of Translational Neuroscience (PXM-2014-014226-000006), Beijing Natural Science Foundation (7132070 and 7141001) and National Natural Science Foundation of China (81771414, 81301015, 31471142 and 31671205).
Competing interests None declared.
Ethics approval The Animal Care and Use Committee of Capital Medical University approved all experimental procedures of this study, which is in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Provenance and peer review Not commissioned; externally peer reviewed.