Article Text
Abstract
Background and purpose 5 recent trials have shown the benefit of endovascular treatment for acute ischaemic stroke (AIS) due to large vessel occlusion of the anterior circulation. This study aims to evaluate the safety and efficacy of Solitaire thrombectomy in patients with moderate-to-severe stroke in the Chinese population, which has a high prevalence of intracranial atherosclerosis.
Methods and analysis This multicentre prospective control study will involve 17 stroke centres in China, and plans to recruit 150 patients in the intervention group, and 150 patients in the medical group, in which patients meet enrolment criteria but refuse intervention. Patients with AIS due to large vessel occlusion indicated for treatment with Solitaire stent retriever within 12 hours of symptom onset, and who meet the inclusion and exclusion criteria, will be enrolled in this study. The primary efficacy endpoint is functional independence as defined by a modified Rankin Scale (mRS) score ≤2 at 90 days or by functional improvement as defined by mRS, using shift analysis. The procedural efficacy endpoint is arterial recanalisation of the occluded target vessel measured by a modified Thrombolysis in Cerebral Infarction (mTICI) score equal or superior to 2b right following the use of the study device. The primary safety endpoint is symptomatic intracranial haemorrhage (sICH) within 24±3 hours postprocedure.
Ethics and dissemination The protocol was approved by the Ethics Committee at the coordinating centre and by the local Institutional Review Board of each participating centre.
Trial registration number NCT02350283.
- Acute Ischemic Stroke
- Mechanical Thrombectomy
- Solitaire Device
- Endovascular therapy
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
Stroke is the leading cause of death in China, responsible for 22.45% of all deaths. Three times as many patients die from stroke as from coronary artery disease. Approximately 65% of Chinese strokes are of ischaemic type.1 Proximal large vessel atherosclerotic stenosis or occlusion accounts for 35–40% of all acute ischaemic strokes (AIS). There is a striking ethnic difference in the distribution of atherosclerosis in the cerebral vasculature of patients with ischaemic stroke. In contrast to the high incidence of extracranial large artery atherosclerosis in the Caucasian population, intracranial atherosclerosis (affecting the middle cerebral artery (MCA), intracranial portion of the internal carotid artery and vertebrobasilar artery) is the predominant cause of ischaemic stroke in the Asian population. In fact, intracranial atherosclerosis is estimated to account for 33–50% of all AIS in the Chinese population.2
Early recanalisation of the occluded cerebral vessel is the key to successful treatment of AIS.3 Before December 2014, the only proven and effective treatment for AIS was recombinant tissue-type plasminogen activator (alteplase).4 Intravenous recombinant tissue plasminogen activator (intravenous alteplase) given up to 3 hours of symptom onset is the only effective therapy for patients with AIS, this is supported by a National Institute of Neurological Disorders and Stroke (NINDS) study.5 Although the European Cooperative Acute Stroke Study III (ECASS III) study demonstrated benefit of alteplase in selected patients up to 4.5 hours after symptom onset, the use of intravenous alteplase is only approved for use within 3 hours, by the Chinese Food and Drug Administration (FDA), and the extended time window is seldom used in China. Owing to lack of public awareness of early stroke symptoms, less than one quarter of patients with stroke arrive in hospital within 3 hours.1 ,6 The delay in patient reporting and limited therapeutic time window, in one study, restricted the use of intravenous alteplase to only 1.6% of patients with acute stroke in China.1 In addition, intravenous alteplase has limited efficacy in successful recanalisation, particularly in patients with large vessel occlusion, where the recanalisation rate is <30%.7 Intra-arterial thrombolysis showed potentially higher recanalisation rate (66.0%) and longer time window (within 6 hour) than intravenous thrombolysis.8 Nonetheless, the relatively high rate of symptomatic intracranial haemorrhage (sICH) (10% vs 2% of placebo) and the long procedural time required for adequate recanalisation are of concern.8
The results of five recently published trials for AIS herald the dawn of a new era in the treatment of patients with large vessel occlusion amenable to endovascular intervention.9–14 Treatment strategies for patients with AIS with large arterial occlusion have now changed, with the publication of the Multicenter Randomized CLinical trial of Endovascular treatment for Acute ischemic stroke in the Netherlands (MR CLEAN),9 Endovascular treatment for Small Core and Anterior circulation Proximal occlusion with Emphasis on minimizing CT to recanalization times (ESCAPE),10 Extending the time for Thrombolysis in Emergency Neurological Deficits—Intra-Arterial (EXTEND IA),11 Solitaire With the Intention For Thrombectomy as PRIMary Endovascular treatment trial (SWIFT PRIME)12 and Randomized Trial of Revascularization with the Solitaire FR Device versus Best Medical Therapy in the Treatment of Acute Stroke due to Anterior Circulation Large Vessel Occlusion Presenting within 8 hours of Symptom Onset (REVASCAT)13 studies. The aforementioned studies have shown consistent and persuasive benefits for intra artery treatment (IAT) using advanced technology in patients with stroke because of intracranial large artery occlusion. Based on the above trials, the American Heart Association/American Stroke Association (AHA/ASA) renewed the evidence-based guidelines for the selection of patients with AIS for endovascular treatment, the endovascular procedure and for systems of care to facilitate endovascular treatment.15 Certain endovascular procedures have been demonstrated to provide clinical benefit in selected patients with AIS. Systems of care should be organised to facilitate the delivery of this care.
However, these trials were carried out in North America and Europe, where cardiac embolism is the most common cause of large vessel strokes. It is unclear if the results of these studies can be reproduced in the Chinese population, which has a high prevalence of intracranial atherosclerosis. No prospective study investigating the efficacy and safety of Solitaire in the Chinese population is available. The Endovascular therapy for Acute ischemic Stroke Trial (EAST) is designed to evaluate the use of Solitaire in patients with moderate-to-severe stroke, within 12 hours of symptom onset, in the Chinese population.
Method/design
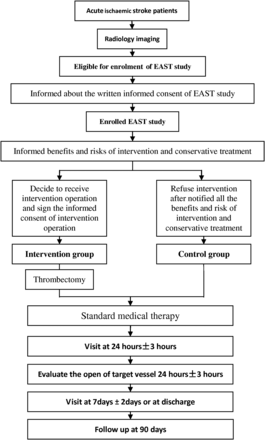
This multicentre prospective trial will involve 17 stroke centres in China, and plans to consecutively recruit 150 patients in the intervention group and 150 patients in the medical group (control). For patients with AIS who are eligible for enrolment, the patient or patient's legally authorised representative will be informed about the written informed consent requirement of the EAST study before enrolment.
After agreement of participation in the EAST study, the patient or patient's legally authorised representative will be further informed of all the benefits and risks of intervention and conservative treatment, by a standardised trained doctor. If the patient or patient's legally authorised representative decides that the patient should receive the intervention operation and signs the informed consent of intervention operation, the patient will be assigned to the intervention group. If the patient participates in the EAST study but refuses intervention after notified of all the benefits and risk of intervention and conservative treatment, the patient will be assigned to the control group.
The intervention patients will be treated for mechanical recanalisation using Solitaire, within 12 hours after stroke onset, plus standard medical therapy. The control arm will be treated with standard medical therapy alone (figure 1).
Endovascular therapy for Acute ischemic Stroke Trial (EAST) study enrolment clinical procedure.
During the trial, multiple indicators will be assessed in all patients at baseline, 24 hour, 7 days (or at discharge, whichever occurs first) and 90 days (see online supplementary I). The Solitaire thrombectomy stent system is provided by Covidien (ev3, Irvine, California, USA). The company will not participate in data collection, analysis, editing, nor in publication strategies. This study is sponsored and conducted by the Cerebrovascular Disease Center of Tiantan Hospital (China), which has the additional responsibility of data analysis. An independent Data and Safety Monitoring Board (DSMB) oversees the conduction, safety and efficacy of the study. The study has been registered—registration number NCT02350283.
Supplemental material
Study status
The study protocol has been approved by the Ethics Committee. Patient recruitment started in January 2015 and will end by June 2016.
Patient population
Intervention group: Any patient with AIS due to large vessel occlusion, indicated for treatment with Solitaire stent retriever within 12 hours of symptoms onset, and meeting the inclusion and exclusion criteria, is eligible (boxes 1 and 2).
Inclusion criteria
Age >18 years
Clinical diagnosis of ischaemic stroke, with stroke symptoms present for at least 30 min and no significant improvement before treatment.
No prestroke functional dependence (prestroke modified Rankin Scale score ≤1).
National Institute of Health Stroke Scale (NIHSS) ≥8 and <30 at the time of enrolment.
Patient can be treated within 12 hours of stroke symptoms onset with minimum of one deployment of the Solitaire device (onset time is defined as the last time the patient was witnessed to be at baseline).
Patient is confirmed to have symptomatic intracranial occlusion, based on single phase, multiphase or dynamic CT angiography/MR angiography or digital subtraction angiography (DSA), at one or more of the following locations: carotid T/L, M1 segment of the middle cerebral artery (MCA), or M2 segment of the MCA equivalent affecting at least 50% of MCA territory.
Patient or patient's legally authorised representative received information about data collection or, if mandatory, has signed and dated an Informed Consent Form.
Exclusion criteria
Baseline non-contrast CT or diffusion weighted imaging (DWI) reveals a moderate/large core defined as extensive early ischaemic changes of Alberta Stroke Program Early CT Score (ASPECTS) 0–6 in the territory of symptomatic intracranial occlusion or DWI lesion volume >50 mL.
Other confirmation of a moderate-to-large core defined in one of three ways:
On a single phase, multiphase or dynamic CT angiography (CTA): no or minimal collaterals in a region greater than 50% of the middle cerebral artery (MCA) territory when compared with pial filling on the contralateral side (multiphase/dynamic CTA preferred);
On CT perfusion (>8 cm coverage): a low cerebral blood volume (CBV) and very low cerebral blood flow (CBF) ASPECTS<6 in the symptomatic MCA territory;
On CT perfusion (<8 cm coverage): a region of low CBV and very low CBF>1/3 of the computed tomography perfusion (CTP)-imaged symptomatic MCA territory.
Groin puncture is not possible within 70 min of the end of CTA/MR angiography acquisition.
Seizure at onset of stroke.
Prior stroke within the past 3 months.
Patient with a pre-existing neurological or psychiatric disease that would confound the neurological and functional evaluations.
Presumed septic embolus or suspicion of bacterial endocarditis.
Life expectancy of <90 days.
Known history of intracranial hemorrhage (ICH), subarachnoid haemorrhage, arteriovenous malformation or tumour.
Known disease with increased bleeding risk during the past 3 months, for example, severe liver disease, ulcerative gastrointestinal disease, oesophageal varices, hepatic failure.
Major surgery, significant trauma or haemorrhagic disease in past 10 days.
Uncompensated hypertension defined as systolic blood pressure >185 mm Hg or diastolic blood pressure ≥110 mm Hg on three repeated measurements at least 10 min apart.
Renal failure as defined by a serum creatinine level >2.0 mg/dl or glomerular filtration rate <30 ml/min*1.73 m2.
Platelet count below 100 000/mm3.
Blood glucose <2.8 or >22.2 mmol/L.
Patients receiving oral anticoagulants, for example, warfarin sodium, and coagulant response time (international normalised ratio) >1.5.
Administration of heparin within the previous 48 hours and activated partial thromboplastin time (APTT) time exceeding the upper limit of normal for laboratory.
Suspected intracranial dissection as a cause of stroke.
Clinical history, past imaging or clinical judgement suggests that the intracranial occlusion is chronic.
No femoral pulses.
Contraindications of DSA examination, severe contrast allergy or absolute contraindication to iodinated contrast.
Pregnancy; if a woman of childbearing potential has a positive urine or serum β-human chorionic gonadotropin test.
Control group: Includes patients who meet enrolment criteria but refuse intervention.
Treatment plan
Interventional treatment with Solitaire
All procedures will be performed via a femoral artery approach, under local anaesthesia. Conscious sedation will be administered as needed.16 The patients will be intubated only if there is a significant risk of airway compromise. General anaesthesia will be induced if the patient is combative and the procedure cannot proceed even with conscious sedation, or conscious sedation is considered too risky given the patient's medical conditions and airway compromise. An 8 Fr sheath, 90 cm (Cook) will be introduced into the femoral access and placed into the affected common carotid artery. When an 8 Fr balloon guiding catheter is available, the balloon catheter will be placed into the cervical internal carotid artery. Without balloon guiding catheter, the long sheath may be placed into the cervical internal carotid artery if feasible, or a 6–8 Fr guiding catheter may be inserted into the internal carotid artery safely, as high as possible. A 0.21-inch internal diameter microcatheter (such as Prowler Select Plus, Cordis or Vasco 21, Balt) will be navigated distal to the point of occlusion over a 0.014-inch steerable microwire. Gentle contrast injection is required to confirm microcatheter position and exclude perforation. After gently flushing out the contrast with saline, a Solitaire device will be deployed through the microcatheter. Control angiography will be performed to evaluate the correct placement and expansion of the device. The device will be left in place for 5–10 min, allowing full expansion of the nitinol stent through the thrombus. The fully deployed Solitaire device together with the delivery microcatheter will be gently pulled back as a single unit and recovered. Before this retrieval, the balloon guide catheter will be inflated and manual aspiration performed with a 50 mL syringe through the haemostatic valve, to reverse the flow in the target artery and therefore reduce the risk of thromboembolism. Without balloon-guiding catheter, aspiration will be performed through the long sheath or the 8 Fr guiding catheter. Successful recanalisation will be defined as modified Thrombolysis in Cerebral Infarction (mTICI) ≥2b in all treatable vessels. If the treated vessel is not opened to at least mTICI 2b with a maximum of five passes of the thrombectomy device, the treatment will be considered a failure. No intra-arterial fibrinolytics will be administered at any point during this study, even if the recanalisation attempt is unsuccessful (figure 2).
Solitaire revascularisation device clinical procedure. BP, blood pressure; CTA, CT angiography; IFU, instruction for use; MRA, MR angiography; mTICI, modified Thrombolysis in Cerebral Infarction; NIHSS, National Institute of Health Stroke Scale.
The following steps will be taken if there is a suspicion of underlying atherosclerosis occluding the vessel. If there is difficulty in passing the 0.21-inch microcatheter through the vessel occlusion despite adequate proximal support, angioplasty with a 2 mm balloon will be allowed to facilitate the passage of the microcatheter. If underlying stenosis is discovered after removal of the Solitaire device, a repeat angiogram will be performed with different views to confirm that the stenosis is not due to vasospasm or dissection. Next, the patient will be prepared for intracranial angioplasty and/or stenting for underlying intracranial atherosclerosis, using 300 mg of rectal aspirin. A cone beam CT will be obtained in the angiography suite, to exclude intracranial haemorrhage. If not feasible, the patient will be subjected to a regular head CT. After excluding intracranial haemorrhage, primary angioplasty will be performed to improve flow to the distal vascular territory and reduce the risks of immediate reocclusion. A residual stenosis of 40–50% is considered acceptable. The Solitaire stent will be detached for stenting of intracranial stenosis only if there is haemodynamically significant recoil or dissection. If the patient received primary angioplasty, 300 mg of loading dose of clopidogrel will be given to the patient orally or via nasogastric tube immediately after the procedure. Intravenous IIb3a inhibitor will be administered immediately before stenting. The stenting patients will be loaded with clopidogrel as well immediately after the procedure and the IIb3a inhibitor infusion will be stopped 2 hours after the loading dose of clopidogrel. All patients indicated for intracranial angioplasty and/or stenting will be treated as intracranial atherosclerosis disease cases and will be included in the subgroup analysis. An MR angiography or CT angiography (CTA) will be obtained after the procedure, to assess the patency of the target vessel (figure 2).
Groin punctures will be routinely closed with an Angio-Seal (St Jude Medical, St Paul, Minnesota, USA). National Institute of Health Stroke Scale (NIHSS) will be noted and blood pressure (BP) assessment carried out immediately after the procedure.
Postprocedure, the patients will be admitted to an intensive care unit. Standard medical therapy will be provided to these patients. BP parameters will be set by the operators and are expected to generally be below 180/100 mm Hg.
Standard medical management
Patients assigned to the medical arm will receive the following medical treatment according to the AHA Guidelines for the Early Management of Patients with Acute Ischemic Stroke.17 ,18 All patients will be admitted to a stroke unit with cardiac monitoring. Patients who received intravenous alteplase will be admitted to an intensive care unit. Airway support, ventilation assistance and supplemental oxygen will be provided to maintain oxygen saturation >94%. Hyperthermia (temperature >38°C) will be treated with antipyretics or physical cooling. The source of hyperthermia will be identified and treated accordingly. Hyperglycaemia and hypoglycaemia will be corrected. Prophylaxis for deep venous thrombosis will be implemented. Swallowing evaluation will be performed within 48 hours before administering patients any oral medication or other intake. BP will be maintained below 180/100 mm Hg. Aspirin (300 mg initial dose) will be started within 24 hours in patients not treated with intravenous alteplase or within 24–48 hours in patients who received intravenous alteplase and were found to have no intracranial haemorrhage on follow-up CT. Patients with low density lipoprotein (LDL)>100 mmol/L will be treated with statin. A check list of the above measures will be provided to the individual centre and treatment team and will be monitored closely to ensure standard medical management and that patients enrolled in the medical arm receive a level of care similar to that received by patients with stroke admitted to primary stroke centres in the USA.
Primary endpoint
The primary efficacy endpoint is functional independence as defined by a modified Rankin Scale (mRS) score ≤2 at 90 days or by functional improvement as defined by mRS using shift analysis. The procedural efficacy endpoint is arterial recanalisation of the occluded target vessel, measured by mTICI score equal or superior to 2b right following the use of the study device. The primary safety endpoint is sICH with 24±3 hours postprocedure.
sICH is defined as parenchymal, subarachnoid or intraventricular haemorrhage detected by CT or MRI, associated with seizures or new neurological symptoms or signs (headache, change in level of consciousness, or focal neurological deficits), lasting at least 24 hours, and is only regarded as a primary endpoint if it occurs within 30 days after revascularisation.
Secondary endpoints
The secondary endpoints are related to the use of the study device and procedure: serious adverse events (SAEs) at 14 days or discharge; volume of cerebral infarction as measured by a CT scan at 24±3 hours postprocedure; arterial reperfusion measured by reperfusion ratio on CT scan 24±3 hours postprocedure; infarction in participants who achieved mTICI 2b-3 reperfusion without intracranial haemorrhage; death due to any cause at 14 days or discharge and at 90 days; change in NIHSS at 24±3 hours postprocedure; change in NIHSS at 14 days or discharge postprocedure; change in NIHSS at 90±7 days; qualify of life at 90±7 days (EuroQol-5 Dimensions, BI=Barthel Index (EQ-5D), BI); the proportion of patients with a safety outcome; proportion of patients with the composite of: (1) sICH, (2) major bleeding due to femoral artery access complications including groin haematoma and retroperitoneal haematoma and (3) contrast nephropathy; economic (cost-effectiveness) analysis; evaluation of waiver/deferral of consent process; total radiation dose (CT, CTA, angiography) reported as a continuous measure; proportion of patients with malignant MCA infarction; proportion of patients undergoing hemicraniectomy; functional independence as defined by mRS score ≤2 at 90 days in patients with intracranial atherosclerosis who received intervention; functional independence as defined by mRS score ≤2 at 90 days in intervention patients excluding intracranial atherosclerosis; arterial recanalisation of the occluded target vessel measured by mTICI score equal or superior to 2b immediately after the use of the study device in patients with intracranial atherosclerosis; mTICI score equal to or better than 2b at 3 hours in patients with intracranial atherosclerosis; mTICI score equal to or better than 2b in intervention patients without intracranial atherosclerosis; sICH within 24±3 hours postprocedure in patients with intracranial atherosclerosis; and sICH within 24±3 hours postprocedure in patients without intracranial atherosclerosis.
Exploratory purpose
The influence of different classifications of the Trial of Org 10 172 in Acute Stroke Treatment (TOAST) on 90 days functional independence, arterial recanalisation, sICH and procedure time of thrombectomy. The influence of different sex on 90 days functional independence, arterial recanalisation, sICH and SAEs. The influence of different anaesthesia type on 90 days functional independence, arterial recanalisation, sICH and procedure time of thrombectomy. The influence of antiplatelet drugs and platelet membrane glycoprotein II b/III a receptor antagonists on 90 days functional independence, arterial recanalisation and sICH. The influence of stenosis of occluded artery on 90 days functional independence, arterial recanalisation, sICH and procedure time of thrombectomy. The influence of baseline BP and glucose on primary results.
Statistical analysis
The primary outcome will be evaluated by a logistic regression model comparing the intervention and medical groups. The following confounding factors will be considered during the analysis: age, sex, stroke severity, stroke subtype, particularly intracranial atherosclerosis, artery of occlusion, infarct volume, blood glucose, BP, medical history, time of start treatment, time of recanalisation, use of antiplatelet therapy, destruction of blood–brain barrier, and biomarkers. Multivariable regressions will be performed adjusting for potential covariates and adjusting for the propensity score.
For secondary outcomes, a logistic regression model will be used as well. Evaluation of the safety assessments and additional measurements during the trial (including treatment interruption/discontinuation, lifestyle evaluation and use of concomitant medications) will be based on appropriate summary statistics. Full details of analyses to be performed will be itemised in a separate Statistical Analysis Plan (SAP). The propensity score model will be used to improve the sensitivity of analysis. A non-parsimonious multivariable logistic regression model will be used to determine the propensity for thrombectomy regardless of the outcome. All the baseline characters will be included to calculate the propensity score. A propensity score, indicating the predicted probability of receiving thrombectomy, will then be calculated from the logistic equation for each patient. The propensity score will then be included along with the comparison variable (thrombectomy or control) in multivariable analyses of outcome producing an adjusted OR with 95% CI.
Strengths and limitations of this study
This multicentre prospective control study will elucidate the safety and efficacy of thrombectomy with Solitaire stent retriever for patients with moderate-to-severe stroke in the Chinese population due to anterior large-vessel occlusion. The thrombectomy will be performed within 12 hours of symptom onset in patients meeting the inclusion and exclusion criteria. However, this study has limitations. It focuses on the Chinese population and requires caution when applying the findings to patients of other ethnicity. The study has inadequate statistical power to assess the impact of the endovascular treatment on major clinical outcomes.
Data and Safety Monitoring Board
DSMB members are independent of the researchers and the steering committee. DSMB is responsible for assuring that study participants are not exposed to unnecessary risks, and that the study is conducted according to high scientific and ethical standards. The DSMB is responsible for advising early termination of the study in the event of unexpected safety concerns or if treatment differences were apparent at the prespecified interim analyses.
Acknowledgments
The authors extend their special thanks to the physicians as well as all other personnel who dedicated themselves to the EAST project.
References
Footnotes
Twitter Follow Yilong Wang at @yilong
Collaborators The EAST investigators include: Steering Committee: Zhongrong Miao (principal investigator); Yongjun Wang (co-principle investigator); Jeffrey L Saver (USA); Joseph P Broderick (USA); Lei Feng (USA); Xingquan Zhao; Liping Liu; Yilong Wang. Executive Committee: Zhongrong Miao; Yongjun Wang; Yilong Wang; Xingquan Zhao; Liping Liu; Xiaoling Liao; Chunjuan Wang. Clinical sites (principal investigator) in order of participants enrolled: Zhongrong Miao (principle investigator, E-mail: zhongrongm@163.com), Beijing Tiantan Hospital; Yongjun Wang (co-principle investigator, email: yongjunwang1962@gmail.com), Beijing Tiantan Hospital; Xiaochuan Huo, Beijing Tiantan Hospital; Xiaoling Liao, Beijing Tiantan Hospital; Xingquan Zhao, Beijing Tiantan Hospital; Chunjuan Wang, Beijing Tiantan Hospital; Liping Liu, Beijing Tiantan Hospital; Yuesong Pan, Beijing Tiantan Hospital; Hao Li, Beijing Tiantan Hospital; Yilong Wang, Beijing Tiantan Hospital; Feng Gao, Beijing Tiantan Hospital; Ning Ma, Beijing Tiantan Hospital; Dapeng Mo, Beijing Tiantan Hospital; Ya Peng, Changzhou No 1 People's Hospital; Hang Lin, Fuzhou PLA General Hospital; Meng Zhang, Daping Hospital; Wei Wu, QiLu Hospital of ShanDong University; Zaiyu Guo, Tianjin Teda Hospital; Li Liu, Chifeng Municipal Hospital; Changchun Jiang, Baotou Central Hospital; Hua Yang, Affiliated Hospital of Guiyang Medical College; Qiyi Zhu, People's Hospital of Linyi City; Xiaoxiang Peng, Hubei Zhongshan Hospital; Yibin Cao, Tangshan Gongren Hospital; Anding Xu, The First Affiliated Hospital of Jinan University; Shengli Chen, Chongqing Sanxia Central Hospital; Cunfeng Song, Liaocheng Third People's Hospital; Liping Wei, Luoyang Central Hospital affiliated to Zhengzhou University; Hui Liang, Yantai Hill Hospital. Data and Safety Monitoring Board: Hao Li, Haipeng Shen, Yong Jiang, Yuesong Pan. Statistical and Data Management Centre: Yilong Wang; Anxin Wang; Gaifen Liu; Xianwei Wang. Events Adjudication Committee: Liebeskind DS (USA); Joseph P Broderick (USA); Lei Feng (USA); Anding Xu; Peiyi Gao; Wengui Yu.
Funding This study is sponsored and conducted by the Cerebrovascular Disease Center of Tiantan Hospital, which is also responsible for data analysis. This study is funded by the National Science and Technology Major Project of China (2011BAI08B02, 2015BAI12B04 and 2015BAI12B02).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.