Key Points
-
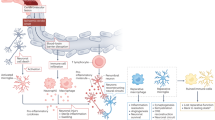
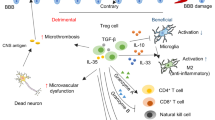
Recognition that immune mechanisms contribute to stroke and an increasing ability to manipulate the immune system have prompted suggestions that immunomodulation could be a feasible therapy for acute stroke
-
Immune interventions—including nonspecific anti-inflammatory drugs and approaches that target immune cells, inflammatory mediators and adhesion molecules—have been tested in patients with acute ischaemic stroke, with mixed outcomes
-
Proof-of-concept studies have demonstrated that fingolimod can attenuate brain inflammation and improve neurological outcomes in patients with acute stroke; a large international trial of natalizumab is nearly complete
-
Trials have shown that drugs that target multiple elements of the immune system, and that act quickly, could be viable candidates
-
Future success of immunomodulation as a therapy for stroke depends on further elucidation of immune interactions during stroke, and on the ability to limit immune-mediated tissue damage and promote tissue repair
Abstract
Approaches for the effective management of acute stroke are sparse, and many measures for brain protection fail. However, our ability to modulate the immune system and modify the progression of multiple sclerosis is increasing. As a result, immune interventions are currently being explored as therapeutic interventions in acute stroke. In this Review, we compare the immunological features of acute stroke with those of multiple sclerosis, identify unique immunological features of stroke, and consider the evidence for immune interventions. In patients with acute stroke, microglial activation and cell death products trigger an inflammatory cascade that damages vessels and the parenchyma within minutes to hours of the ischaemia or haemorrhage. Immune interventions that restrict brain inflammation, vascular permeability and tissue oedema must be administered rapidly to reduce acute immune-mediated destruction and to avoid subsequent immunosuppression. Preliminary results suggest that the use of drugs that modify disease in multiple sclerosis might accomplish these goals in ischaemic and haemorrhagic stroke. Further elucidation of the immune mechanisms involved in stroke is likely to lead to successful immune interventions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Albers, G. W. et al. Intravenous tissue-type plasminogen activator for treatment of acute stroke: the Standard Treatment with Alteplase to Reverse Stroke (STARS) study. JAMA 283, 1145–1150 (2000).
Lees, K. R. et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 375, 1695–1703 (2010).
Fonarow, G. C. et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA 311, 1632–1640 (2014).
Prabhakaran, S., Ruff, I. & Bernstein, R. A. Acute stroke intervention: a systematic review. JAMA 313, 1451–1462 (2015).
Goyal, M. et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 372, 1019–1030 (2015).
Campbell, B. C. et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 372, 1009–1018 (2015).
Kidwell, C. S. et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N. Engl. J. Med. 368, 914–923 (2013).
Broderick, J. P. et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N. Engl. J. Med. 368, 893–903 (2013).
Ciccone, A. et al. Endovascular treatment for acute ischemic stroke. N. Engl. J. Med. 368, 904–913 (2013).
Berkhemer, O. A. et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 372, 11–20 (2015).
O'Collins, V. E. et al. 1,026 experimental treatments in acute stroke. Ann. Neurol. 59, 467–477 (2006).
Macrez, R. et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 10, 471–480 (2011).
Iadecola, C. & Anrather, J. The immunology of stroke: from mechanisms to translation. Nat. Med. 17, 796–808 (2011).
Wang, J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog. Neurobiol. 92, 463–477 (2010).
Chamorro, A. et al. The immunology of acute stroke. Nat. Rev. Neurol. 8, 401–410 (2012).
Mracsko, E. & Veltkamp, R. Neuroinflammation after intracerebral hemorrhage. Front. Cell. Neurosci. 8, 388 (2014).
Fu, Y. et al. Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA Neurol. 71, 1092–1101 (2014).
Fu, Y. et al. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc. Natl Acad. Sci. USA 111, 18315–18320 (2014).
Elkind, M. S. Inflammatory mechanisms of stroke. Stroke 41, S3–S8 (2010).
Marnane, M. et al. Plaque inflammation and unstable morphology are associated with early stroke recurrence in symptomatic carotid stenosis. Stroke 45, 801–806 (2014).
Courties, G., Moskowitz, M. A. & Nahrendorf, M. The innate immune system after ischemic injury: lessons to be learned from the heart and brain. JAMA Neurol. 71, 233–236 (2014).
Hao, J. et al. Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J. Exp. Med. 207, 1907–1921 (2010).
Hao, J. et al. Interleukin-2/interleukin-2 antibody therapy induces target organ natural killer cells that inhibit central nervous system inflammation. Ann. Neurol. 69, 721–734 (2011).
Stys, P. K., Zamponi, G. W., van Minnen, J. & Geurts, J. J. Will the real multiple sclerosis please stand up? Nat. Rev. Neurosci. 13, 507–514 (2012).
Lucas, S. M., Rothwell, N. J. & Gibson, R. M. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 147 (Suppl. 1), S232–S240 (2006).
Chen, G. Y. & Nunez, G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837 (2010).
Gan, Y. et al. Ischemic neurons recruit natural killer cells that accelerate brain infarction. Proc. Natl Acad. Sci. USA 111, 2704–2709 (2014).
Gelderblom, M. et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40, 1849–1857 (2009).
Hammond, M. D. et al. CCR2+ Ly6Chi inflammatory monocyte recruitment exacerbates acute disability following intracerebral hemorrhage. J. Neurosci. 34, 3901–3909 (2014).
Hammond, M. D., Ambler, W. G., Ai, Y. & Sansing, L. H. alpha4 integrin is a regulator of leucocyte recruitment after experimental intracerebral hemorrhage. Stroke 45, 2485–2487 (2014).
Liesz, A. et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med. 15, 192–199 (2009).
Allan, S. M., Tyrrell, P. J. & Rothwell, N. J. Interleukin-1 and neuronal injury. Nat. Rev. Immunol. 5, 629–640 (2005).
Lambertsen, K. L. et al. A role for interferon-gamma in focal cerebral ischemia in mice. J. Neuropathol. Exp. Neurol. 63, 942–955 (2004).
Seifert, H. A. et al. Pro-inflammatory interferon gamma signalling is directly associated with stroke induced neurodegeneration. J. Neuroimmune Pharmacol. 9, 679–689 (2014).
Yilmaz, G., Arumugam, T. V., Stokes, K. Y. & Granger, D. N. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation 113, 2105–2112 (2006).
Doyle, K. P. et al. B-lymphocyte-mediated delayed cognitive impairment following stroke. J. Neurosci. 35, 2133–2145 (2015).
Becker, K. J. et al. Autoimmune responses to the brain after stroke are associated with worse outcome. Stroke 42, 2763–2769 (2011).
Romer, C. et al. Blocking stroke-induced immunodeficiency increases CNS antigen-specific autoreactivity but does not worsen functional outcome after experimental stroke. J. Neurosci. 35, 7777–7794 (2015).
Meisel, C. & Meisel, A. Suppressing immunosuppression after stroke. N. Engl. J. Med. 365, 2134–2136 (2011).
Sacco, R. L. et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N. Engl. J. Med. 359, 1238–1251 (2008).
Amarenco, P. et al. High-dose atorvastatin after stroke or transient ischemic attack. N. Engl. J. Med. 355, 549–559 (2006).
Zhang, R. L. et al. Anti-ICAM-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in the rat. Neurology 44, 1747–1751 (1994).
Enlimomab Acute Stroke Trial, I. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology 57, 1428–1434 (2001).
Furuya, K. et al. Examination of several potential mechanisms for the negative outcome in a clinical stroke trial of enlimomab, a murine anti-human intercellular adhesion molecule-1 antibody: a bedside-to-bench study. Stroke 32, 2665–2674 (2001).
Emsley, H. C. et al. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J. Neurol. Neurosurg. Psychiatry 76, 1366–1372 (2005).
Shyu, K. G., Chang, H. & Lin, C. C. Serum levels of intercellular adhesion molecule-1 and E-selectin in patients with acute ischemic stroke. J. Neurol. 244, 90–93 (1997).
US National Library of Medicine. ClinicalTrials.gov[online] (2010).
US National Library of Medicine. ClinicalTrials.gov[online] (2015).
Lampl, Y. et al. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology 69, 1404–1410 (2007).
Fagan, S. C. et al. Minocycline to improve neurologic outcome in stroke (MINOS): a dose-finding study. Stroke 41, 2283–2287 (2010).
US National Library of Medicine. ClinicalTrials.gov[online] (2013).
Schwab, S. R. et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309, 1735–1739 (2005).
Massberg, S. & von Andrian, U. H. Fingolimod and sphingosine-1-phosphate—modifiers of lymphocyte migration. N. Engl. J. Med. 355, 1088–1091 (2006).
Cannon, R. E., Peart, J. C., Hawkins, B. T., Campos, C. R. & Miller, D. S. Targeting blood-brain barrier sphingolipid signalling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc. Natl Acad. Sci. USA 109, 15930–15935 (2012).
Cohen, J. A. & Chun, J. Mechanisms of fingolimod's efficacy and adverse effects in multiple sclerosis. Ann. Neurol. 69, 759–777 (2011).
Budde, K. et al. First human trial of FTY720, a novel immunomodulator, in stable renal transplant patients. J. Am. Soc. Nephrol. 13, 1073–1083 (2002).
Wei, Y. et al. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann. Neurol. 69, 119–129 (2011).
Zhu, Z. et al. Combination of an immune modulator fingolimod with alteplase in acute ischemic stroke: a randomized multi-centre study. Circulation (in press). http://dx.doi.org/10.1161/CIRCULATIONAHA.115.016371.
Liesz, A. et al. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain 134, 704–720 (2011).
Langhauser, F. et al. Blocking of α4 integrin does not protect from acute ischemic stroke in mice. Stroke 45, 1799–1806 (2014).
Schabitz, W. R. & Dirnagl, U. Are we ready to translate T-cell transmigration in stroke? Stroke 45, 1610–1611 (2014).
US National Library of Medicine. ClinicalTrials.gov[online] (2015).
Liu, X. et al. Cell based therapies for ischemic stroke: from basic science to bedside. Prog. Neurobiol. 115, 92–115 (2014).
Moniche, F. et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke: a pilot clinical trial. Stroke 43, 2242–2244 (2012).
Hess, D. C. et al. A double-blind placebo-controlled clinical evaluation of MultiStem for the treatment of ischemic stroke. Int. J. Stroke 9, 381–386 (2014).
van Asch, C. J., Oudendijk, J. F., Rinkel, G. J. & Klijn, C. J. Early intracerebral hematoma expansion after aneurysmal rupture. Stroke 41, 2592–2595 (2010).
Steiner, T. et al. Recommendations for the management of intracranial hemorrhage—part I: spontaneous intracerebral hemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc. Dis. 22, 294–316 (2006).
Trabert, J. & Steiner, T. Deep vein thrombosis and lung embolisms in patients with stroke: prevention and therapy [German]. Nervenarzt 85, 1315–1325 (2014).
Mayer, S. A. & Rincon, F. Treatment of intracerebral haemorrhage. Lancet Neurol. 4, 662–672 (2005).
Sutherland, G. R. & Auer, R. N. Primary intracerebral hemorrhage. J. Clin. Neurosci. 13, 511–517 (2006).
Graham, D. I., McIntosh, T. K., Maxwell, W. L. & Nicoll, J. A. Recent advances in neurotrauma. J. Neuropathol. Exp. Neurol. 59, 641–651 (2000).
Lusardi, T. A., Wolf, J. A., Putt, M. E., Smith, D. H. & Meaney, D. F. Effect of acute calcium influx after mechanical stretch injury in vitro on the viability of hippocampal neurons. J. Neurotrauma 21, 61–72 (2004).
Qureshi, A. I. et al. Extracellular glutamate and other amino acids in experimental intracerebral hemorrhage: an in vivo microdialysis study. Crit. Care Med. 31, 1482–1489 (2003).
Wang, J. & Dore, S. Inflammation after intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 27, 894–908 (2007).
Aronowski, J. & Hall, C. E. New horizons for primary intracerebral haemorrhage treatment: experience from preclinical studies. Neurol. Res. 27, 268–279 (2005).
Lin, S. et al. Haem activates TLR4-mediated inflammatory injury via MyD88/TRIF signalling pathway in intracerebral hemorrhage. J. Neuroinflammation 9, 46 (2012).
Matsushita, H. et al. Suppression of CXCL2 upregulation underlies the therapeutic effect of the retinoid Am80 on intracerebral hemorrhage in mice. J. Neurosci. Res. 92, 1024–1034 (2014).
Li, N. et al. Association of molecular markers with perihematomal edema and clinical outcome in intracerebral hemorrhage. Stroke 44, 658–663 (2013).
Schellinger, P. D. et al. Stroke MRI in intracerebral hemorrhage: is there a perihemorrhagic penumbra? Stroke 34, 1674–1679 (2003).
Zazulia, A. R. et al. Hypoperfusion without ischemia surrounding acute intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 21, 804–810 (2001).
Tapia-Perez, H. et al. Use of statins for the treatment of spontaneous intracerebral hemorrhage: results of a pilot study. Cent. Eur. Neurosurg. 70, 15–20 (2009).
Flint, A. C. et al. Effect of statin use during hospitalization for intracerebral hemorrhage on mortality and discharge disposition. JAMA Neurol. 71, 1364–1371 (2014).
Chu, K. et al. Celecoxib induces functional recovery after intracerebral hemorrhage with reduction of brain edema and perihematomal cell death. J. Cereb. Blood Flow Metab. 24, 926–933 (2004).
Lee, S. H. et al. Effects of celecoxib on hematoma and edema volumes in primary intracerebral hemorrhage: a multicentre randomized controlled trial. Eur. J. Neurol. 20, 1161–1169 (2013).
US National Library of Medicine. ClinicalTrials.gov[online] (2013).
Wu, J. et al. Minocycline attenuates brain edema, brain atrophy and neurological deficits after intracerebral hemorrhage. Acta Neurochir. Suppl. 106, 147–150 (2010).
Szymanska, A., Biernaskie, J., Laidley, D., Granter-Button, S. & Corbett, D. Minocycline and intracerebral hemorrhage: influence of injury severity and delay to treatment. Exp. Neurol. 197, 189–196 (2006).
Wasserman, J. K. & Schlichter, L. C. Minocycline protects the blood-brain barrier and reduces edema following intracerebral hemorrhage in the rat. Exp. Neurol. 207, 227–237 (2007).
US National Library of Medicine. ClinicalTrials.gov[online] (2015).
Rolland, W. B. et al. Fingolimod reduces cerebral lymphocyte infiltration in experimental models of rodent intracerebral hemorrhage. Exp. Neurol. 241, 45–55 (2013).
Rolland, W. B. 2nd et al. FTY720 is neuroprotective and improves functional outcomes after intracerebral hemorrhage in mice. Acta Neurochir. Suppl. 111, 213–217 (2011).
Li, Y. J. et al. Fingolimod alters inflammatory mediators and vascular permeability in intracerebral hemorrhage. Neurosci. Bull. http://dx.doi.org/10.1007/s12264-015-1532-2.
Ajmo, C. T. Jr et al. Blockade of adrenoreceptors inhibits the splenic response to stroke. Exp. Neurol. 218, 47–55 (2009).
Sahota, P. et al. Changes in spleen size in patients with acute ischemic stroke: a pilot observational study. Int. J. Stroke 8, 60–67 (2013).
Hug, A. et al. Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke 40, 3226–3232 (2009).
Mracsko, E. et al. Differential effects of sympathetic nervous system and hypothalamic-pituitary-adrenal axis on systemic immune cells after severe experimental stroke. Brain Behav. Immun. 41, 200–209 (2014).
Wong, C. H., Jenne, C. N., Lee, W. Y., Leger, C. & Kubes, P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science 334, 101–105 (2011).
Prass, K. et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J. Exp. Med. 198, 725–736 (2003).
Westendorp, W. F., Nederkoorn, P. J., Vermeij, J. D., Dijkgraaf, M. G. & van de Beek, D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 11, 110 (2011).
Dziedzic, T., Slowik, A., Pera, J. & Szczudlik, A. Beta-blockers reduce the risk of early death in ischemic stroke. J. Neurol. Sci. 252, 53–56 (2007).
Chamorro, A. et al. The Early Systemic Prophylaxis of Infection After Stroke study: a randomized clinical trial. Stroke 36, 1495–1500 (2005).
Harms, H. et al. Preventive antibacterial therapy in acute ischemic stroke: a randomized controlled trial. PLoS ONE 3, e2158 (2008).
van de Beek, D. et al. Preventive antibiotics for infections in acute stroke: a systematic review and meta-analysis. Arch. Neurol. 66, 1076–1081 (2009).
Westendorp, W. F. et al. The Preventive Antibiotics in Stroke Study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet 385, 1519–1526 (2015).
BioMed Central. ISRCTN registry[online] (2014).
Zhang, L. et al. Estrogen stimulates microglia and brain recovery from hypoxia-ischemia in normoglycemic but not diabetic female mice. J. Clin. Invest. 113, 85–95 (2004).
Neumann, J. et al. Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege. J. Neurosci. 28, 5965–5975 (2008).
Yousry, T. A. et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N. Engl. J. Med. 354, 924–933 (2006).
Bloomgren, G. et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N. Engl. J. Med. 366, 1870–1880 (2012).
Trampe, A. K. et al. Anti-JC virus antibodies in a large German natalizumab-treated multiple sclerosis cohort. Neurology 78, 1736–1742 (2012).
Ribeiro, M. J. et al. Could 18F-DPA-714 PET imaging be interesting to use in the early post-stroke period? EJNMMI Res. 4, 28 (2014).
Santillo, A. F. et al. In vivo imaging of astrocytosis in Alzheimer's disease: an 11C-L-deuteriodeprenyl and PIB PET study. Eur. J. Nucl. Med. Mol. Imaging 38, 2202–2208 (2011).
Doerfler, A. et al. MR contrast agents in acute experimental cerebral ischemia: potential adverse impacts on neurologic outcome and infarction size. J. Magn. Reson. Imaging 11, 418–424 (2000).
Rausch, M. et al. Dynamic patterns of USPIO enhancement can be observed in macrophages after ischemic brain damage. Magn. Reson. Med. 46, 1018–1022 (2001).
Saleh, A. et al. In vivo MRI of brain inflammation in human ischaemic stroke. Brain 127, 1670–1677 (2004).
Chamorro, A., Urra, X. & Planas, A. M. Infection after acute ischemic stroke: a manifestation of brain-induced immunodepression. Stroke 38, 1097–1103 (2007).
Hao, J. et al. Nicotinic receptor β2 determines NK cell-dependent metastasis in a murine model of metastatic lung cancer. PLoS ONE 8, e57495 (2013).
Acknowledgements
The authors would like to thank members of their laboratories and clinical teams for their passionate work in immunology and stroke. They would also like to thank C. Iadecola (Feil Family Brain and Mind Research Institute, Weill Cornell Medical College, New York, NY, USA), D. Huang (Neurology and Neuroscience Associates, Unity Health Network, Akron, OH, USA), T. Vollmer (Department of Neurology, University of Colorado School of Medicine, Aurora, CO, USA) and A. La Cava (Department of Medicine, University of California at Los Angeles, CA, USA) for fruitful discussions and advice, and P. Minick and K. Wood (Department of Neurology, Barrow Neurological Institute, Phoenix, AZ, USA) for editorial assistance. The authors' research programmes are supported in part by the National Basic Research Program of China (2013CB966900), National Natural Science Foundation of China (81230,028), National Key Clinical Speciality Construction Project of China, NIH (R01AI083294, R01NS34179 and R01NS081179), the American Heart Association (14GRNT18970031) and the Foundation Leducq. This paper is dedicated to our families.
Author information
Authors and Affiliations
Contributions
Y.F. and F.-D.S. researched data for the article, and Y.F., Q.L., J.A. and F.-D.S. wrote the article. F.-D.S. made a substantial contribution to the discussion of content, and all authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Fu, Y., Liu, Q., Anrather, J. et al. Immune interventions in stroke. Nat Rev Neurol 11, 524–535 (2015). https://doi.org/10.1038/nrneurol.2015.144
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2015.144
This article is cited by
-
The role of stroke-induced immunosuppression as a predictor of functional outcome in the neurorehabilitation setting
Scientific Reports (2024)
-
Low neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict favorable outcomes after endovascular treatment in acute basilar artery occlusion: subgroup analysis of the BASILAR registry
BMC Neurology (2023)
-
Natural killer cells in the central nervous system
Cell Communication and Signaling (2023)
-
Nanoparticles Treat Ischemic Stroke by Responding to Stroke Microenvironment
BioNanoScience (2023)
-
Dihydromyricetin Alleviates Ischemic Brain Injury by Antagonizing Pyroptosis in Rats
Neurotherapeutics (2023)