Article Text
Abstract
Migraines are generally considered a relatively benign neurological condition. However, research has shown an association between migraines and stroke, and especially between migraine with aura and ischaemic stroke. Patients can also suffer from migrainous infarction, a subset of ischaemic stroke that often occurs in the posterior circulation of younger women. The exact pathogenesis of migrainous infarct is not known, but it is theorised that the duration and local neuronal energy level from cortical spreading depression may be a key factor. Other factors contributing to migrainous infarct may include vascular, inflammatory, endothelial structure, patent foramen ovale, gender, oral contraceptive pill use and smoking. Vasoconstrictors such as the triptan and ergot class are commonly used to treat migraines and may also play a role. Migraine is also shown to be correlated to haemorrhagic stroke, although studies do not demonstrate causation versus association, and further studies are warranted. There are also some rare genetic diseases such as cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy, retinal vasculopathy with cerebral leukodystrophy and others, which can cause both migraines and infarcts. On imaging, many migraineurs are found to have white matter changes similar to those seen in patients with stroke. These may be caused in part by alterations in resting cerebral blood flow and vasoconstrictor use. In treating patients with migraines, it is important to identify and modify any vascular risk factors such as hypertension, smoking, oral contraceptive pill use and lifestyle factors. Further studies will determine if more aggressive treatment of migraines can ultimately lead to fewer strokes in this population.
- migraine
- migraine with aura
- ischemic/hemorrhagic stroke
- migrainous infarction
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
With the increase in both size and age of the world population, stroke has become one of the heaviest global health burdens, especially in middle-income to low-income countries. Based on data from the Global Burden of Disease Study 2013, more than 10 million people suffer an acute stroke (ischaemic or haemorrhagic) each year, which represents a global incidence of 175.4/100 000/year.1 In the USA, stroke is the fifth leading cause of death and approximately 8 00 000 people have a stroke each year.2 3 Though migraine is generally considered a benign disease that affects 11.7% of the US population (17.1% of women and 5.6% of men),4 there is unequivocal evidence showing association between migraine and stroke. The association between ischaemic stroke and migraine with aura, especially in younger (age ≤45 years) women, is already established and well accepted,5–8 but there is still uncertainty with respect to its mechanisms, correlation between stroke and migraine without aura, management, prophylaxis in clinical practice and so on. However, these two conditions are known to have in common the following: (1) pathogenesis, (2) risk factors and (3) clinical and imaging manifestation.
In this review, we will discuss epidemiology, possible mechanisms, advanced genetic findings and clinical management of this complex situation. Hopefully, with the advance of genetic research, genome-wide screening and knowledge for specific diseases, such as cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), retinal vasculopathy with cerebral leukodystrophy (RVCL) and others, one will be able to use individualised prophylaxis and treatment for patients and begin the era of precision medicine.
Clinical presentation of migraine and stroke
Migraine is one of the most common neurological disorders, affecting 11%–15% of the population. It is best described as a moderate to severe throbbing headache lasting 4–72 hours and is often associated with nausea, vomiting, photophobia and phonophobia. About one-third of migraineurs experience auras including speech, sensory, visual or motor symptoms, which can mimic many common stroke-like symptoms. Migraine and stroke can have similar symptoms, especially migraine variants such as basilar migraine, ocular migraine and others.
Ischaemic strokes comprise about 80% of all strokes and are a result of reduced blood flow to the brain causing damage and death to brain tissue. They can occur due to a variety of reasons such as large artery atherosclerosis, embolism, small vessel occlusion or decreased systemic perfusion. Ischaemic strokes can have variable presentations depending on which cerebral vessels have been affected. For example, ischemia in the vertebrobasilar arteries can lead to cranial nerve palsies, ataxia, diplopia, dizziness, nausea, vomiting, dysarthria or dysphagia while ischemia in the anterior cerebral artery could lead to motor and/or sensory deficits.
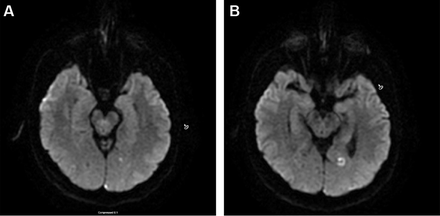
Ischaemic stroke in a migraine sufferer may be categorised as cerebral infarction of other cause coexisting with migraine, cerebral infarction of other cause presenting with symptoms resembling migraine with aura (MA) or cerebral infarction occurring during the course of a typical migraine with aura attack. Only the last fulfils criteria for migrainous infarction. Migrainous infarction mostly occurs in the posterior circulation and in younger women (figure 1A,B).
Typical manifestation of migrainous infarct on MRI diffusion weighted imaging in a 45-year-old female patient with chronic migraine with aura.
Diagnosis of migrainous infarction is based on International Classification of Headache Disorders (http://www.ichd-3.org)
Description
One or more migraine aura symptoms associated with an ischaemic brain lesion in the appropriate territory demonstrated by neuroimaging.
Diagnostic criteria
A migraine attack fulfilling criteria B and C
Occurring in a patient with migraine with aura and typical of previous attacks except that one or more aura symptoms persists for >60 min
Neuroimaging demonstrates ischaemic infarction in a relevant area
Not better accounted for by another diagnosis.
Haemorrhagic strokes, which can quickly escalate into a devastating clinical situation, make up about 12% of all strokes. Some risk factors have been identified such as hypertension, aneurysm and smoking, but much remains to be discovered about their triggers and prevention. Among those, migraine has been suspected as a risk factor for haemorrhagic stroke. The association between migraine and haemorrhagic stroke, including intracerebral haemorrhage and subarachnoid haemorrhage, is weaker than its relation with ischaemic stroke due to inconsistent data from past decades. A large-scale, population-based, age-matched and sex-matched follow-up study in Taiwan concluded that migraineurs had an increased risk of developing haemorrhagic strokes.6 In the Harvard study, 85 confirmed haemorrhagic strokes were found during a mean follow-up of 13.6 years. After adjustment for age, there was a change between women without migraine and women with MA; HR 2.31 and p=0.0190.9 On the contrary, in a subanalysis on migraine and cardiovascular disease based on three studies by Schürks and colleagues,10 no increased risk for haemorrhagic stroke was demonstrated. A recent meta-analysis in 2013 identified 8 studies (4 case–control and 4 cohort studies) involving a total of 1600 haemorrhagic strokes, which showed that the risk of haemorrhagic stroke was greater in females with any migraine (1.55; 95% CI, 1.16 to 2.07; p=0.003) and in female migraineurs aged less than 45 years (1.57; 95% CI, 1.10 to 2.24; p=0.012).5 Though these available studies bring to light the correlation between migraine and haemorrhagic stroke, they cannot address the question of causation versus association. Therefore, further studies are needed to illustrate the haemorrhagic stroke risk according to migraine type, age, sex and haemorrhagic stroke type.
New development discussion
Pathogenesis
The exact mechanisms for migraine-induced stroke have yet to be determined, although several factors have been extrapolated (box 1). The basis for migraine pathology appears to be neuronal excitability caused by cortical spreading depression (CSD). Spreading depolarisation causes near-complete breakdown of neuronal ion homeostasis and contributes significantly to the higher vulnerability of neurons to ischaemic stress compared with other cells of the body.11 Spreading depolarisation leads to hyperexcitability, followed by spreading depression. The duration of depolarisation and the local neuronal energy level are important factors. High energy levels can lead to spreading depression, while low energy levels (or low perfusion) lead to non-spreading depression and cell death. CSD is associated with excitatory amino acid (eg, glutamate) release, which is known to be involved in ischaemic neuronal injury.
Possible mechanisms of migraine-induced stroke
Cortical spreading depression
Cerebral blood flow hemodynamic change
Increased vascular resistance
Breakdown of neuronal ion homeostasis
Inflammatory factors
CSD related release of neuronal inflammatory mediators
Cytotoxic cell damage due to glutamate release and excessive calcium accumulation
Persistent neurologic deficit due to neuronal necrosis
Endothelia dysfunction
Reduction in bioavailability of vasodilators
Mediated by increased oxidative stress
Preceding development of atherothrombosis
Reduced endothelia repair capacity
Vascular factors
Arterial dissection/spontaneous cervical artery dissection
Increase serum elastase
Patent foramen ovale (controversial)
Genetics
Methylenetetrahydrofolate reductase (MTHFR) C677T
Angiotensin-converting enzyme (ACE)-DD polymorphism
Likely no association with FHM1/CACNA1A, FHM2/ATP1A2, FHM3/SCN1A
Coagulation factors
Increased platelet activation factor
Von Willebrand factor
Protein S deficiency
Others
Medications: ergotamine likely (+), triptan (–)
Antiphospholipid antibody syndrome
FHM: Familial Hemiplegic Migraine
+association found
-no association found
Vascular factors play a role in both migraine and its induced ischaemic stroke. In fact, migraine is suggested as a predisposing condition for spontaneous cervical artery dissection; some have suggested a generalised vascular disorder as a predisposing condition for both diseases.12 13 Endothelial dysfunction and reduced endothelial repair14–16 are hypothesised as contributing factors although the exact mechanism of ischaemic stroke risk in migraine remains unknown. In a case-control study, Liman et al found that female migraineurs with aura (MA) had significantly higher endothelial microparticles (EMPs) from peripheral blood while monocytic and platelet microparticles were increased in all MA patients as well.17 Since higher levels of EMPs have been shown to be related to reduce peripheral endothelial function,18 monitoring EMPs may be a reasonable method to monitor migraineurs’ endothelial function and provide a basis for early intervention. To interpret the mechanisms behind this phenomenon, the authors further conducted one post hoc analysis, which concluded that women with MA had lower levels of stromal cell-derived factor-1 alpha, an important modulator maintaining endothelial integrity via mobilisation of vascular stem cells.19 Last year, investigators from Harvard did a whole genome sequence in 59 674 patients with migraine and 3 16 078 controls. They identified 38 new susceptibility loci for migraine that code for vascular and smooth muscle tissue, which indicates that endothelium plays a very important role in the pathophysiology of migraine.17 These findings strengthened the crucial role of endothelial dysfunction and its correlation with development of stroke in such patients.
For years, researchers have been looking for underlying mechanisms of increased risk of stroke in women, especially for those who are premenopausal and without recognised cardiovascular risk factors.10 Studies have shown a link of migraine, and especially aura, to increased levels of estradiol (eg, oral contraceptive pill use, pregnancy), thrombocytosis and erythrocytosis, von Willebrand factor antigen, fibrinogen, tissue plasminogen activator antigen and endothelial microparticles.18 Hypercoagulability induced by these changes could explain the increased risk of stroke for this specific population of young female migraineurs. However, the exact mechanisms linking hypercoagulability to migraine are not yet explored. One theory is that micro-embolus following platelet aggregation could cause either migraine aura or transient ischaemic attack (TIA), and that migraine aura represents a TIA equivalent.19 This can, to some extent, be demonstrated by increased fibrinogen, D-dimer and galectin-3 levels in patients with migraine.20 In addition, experimental work in rodent models shows that hypoperfusion caused by injections of air, cholesterol crystals and microspheres could trigger CSD without infarction.21 Other theories suggest that CSD may lead to weakening of the blood–brain barrier and endothelial damage, or that migraine may be linked to hypercoagulability through stress.18
Over the past few years, evidence from genetic influence on the migraine–stroke relation emerges, with genetic predisposition playing an important role in the occurrence of both migraine and ischaemic stroke. For example, Kutai et al 22 showed a high incidence of F5 A1691G and F2 G20210A in a group of paediatric patients with migraine and significantly increased factor VIII activity in 25% of the same group of patients from a population of Jewish origin. Therefore, the author recommended thrombophilia survey in patients with migraine and indicated more attention to the shift towards hypercoagulation either during or between migraine attacks.
Independent risk factors of ischaemic stroke in migraineurs include migraine with aura, women, age <45 years, oral contraceptive use and smoking.10 23 Risk factors specific to migraineurs may include migraine-specific medications including triptans and ergot alkaloids. These potent vasoconstrictors may lead to increased blood pressures and thus increase the risk of ischaemic stroke.24
The association between PFO and migraine is currently unknown. Both migraine and PFO are common in the general population.25 ‘Postmortem of 956 adults showed an overall PFO incidence of 27.3%’.26 There are several proposed mechanisms for migraine due to the presence of PFO. Due to the nature of the PFO shunt, subclinical emboli and metabolites from the venous system can circumvent the lungs and directly enter the systemic circulation, potentially irritating the trigeminal nerve and vasculature near the brain, triggering a migraine’.27 The PFO may also cause transient hypoxia, which could lead to subclinical infarcts in the brain that lead to irritation and propensity for migraine.28
There are many studies that explore the relationship between migraine and PFO, but their data is varied and inconclusive. Several older, observational studies report an association between migraine and PFO, especially migraine with aura and PFO.29 Results from these studies have not always been replicated, however, and new studies report conflicting data.30 The first reported association between these two was published in 1998 31. More recently, studies have detected no link between PFO prevalence and migraine with aura.25 32 33 For example, in the study conducted by Larossa et al, 30 they ‘found no differences in the prevalence of PFO in patients with chronic migraine versus episodic migraine patients. Therefore, the presence of PFO does not seem to be related with the frequency of migraine attacks’. Limitations from earlier research include low-powered studies, placebo effect, lack of control group and different patient characteristics.25 In addition, ‘the overall quality of these observational studies was poor’.34 Treatment with aspirin, which is standard after PFO closure, could also be a confounding factor.25
There have been a few randomised studies exploring the migraine–PFO link. Only one trial, the Migraine Intervention With STARFlex Technology (MIST), used a sham procedure to account for placebo effect. The MIST trial is also the only trial that excluded patients with other indications for PFO closure.35 The MIST trial, along with PREMIUM (Prospective, Randomized Investigation to Evaluate Incidence of Headache Reduction in Subjects With Migraine and PFO Using the AMPLATZER PFO Occluder to Medical Management) and PRIMA (Percutaneous Closure of PFO in Migraine with Aura) trials showed negative results for PFO closure as migraine treatment.36 ‘PFO closure did not reduce overall monthly migraine days’.37
Based on the above evidence, closure of PFO is not currently recommended as treatment for migraines. Patients with refractory migraines with frequent auras and significant disability due to migraine may be an exception to these guidelines after careful consideration and exploration with their neurologist.34
Genetic factors
There are certain genetic diseases that include migraines in their clinical presentation and also cause vascular damage, leading to an increased risk of ischaemic stroke (table 1). While these diseases are rare, it is important to understand their clinical presentation and diagnostic findings so they can be included in the differential for migraine patients, especially those with a family history of stroke or dementia. In fact, ‘there is accumulating evidence for a shared genetic basis of migraine and vascular disorders’.38
Genetic factors in migraine–stroke association
The most well known of these is CADASIL. It was the first known genetic locus for stroke, with pathogenic mutations in the NOTCH3 gene.39 Migraine is a predominant feature; it is present in 75.3% of patients and the presenting feature in 67.7%.40 89.9% of CADASIL patients experience migraine with aura, although the pattern differs from that of the general migraine population due to predominance of a prolonged and complicated aura40 with a later age of onset.41 Other clinical features include lacunar strokes and cognitive impairment, and these patients present with stroke at a young age, with the median age at onset of 48 years40. Characteristic involvement of the anterior temporal pole is highly sensitive and specific for CADASIL.42 On electron microscope, granular osmiophilic material can be seen around the smooth muscle cells of blood vessels.43
Cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy is related to mutations in the HTRA1 gene44 and is a rare disease. It has been described in 50 patients, mostly Japanese and Chinese,44 but more recently in some Europeans.45 Patients have migraine more rarely and are noted to have recurrent lacunar infarcts (mainly in basal ganglia or brainstem), progressive cognitive and motor impairment, seizures and psychiatric disturbances. They also develop non-neurological symptoms including early-onset diffuse alopecia and degenerative disc disease resulting in acute middle to lower back pain. The disease progresses rapidly, and patients develop dementia and become bedridden around 40 years44. MRI shows white matter hyperintensities, which often precede symptom onset. They are generally in the deep white matter and periventricular regions and sparing the subcortical arcuate fibres as seen in CADASIL.44
RVCL is an autosomal dominant disease of the small vessels, caused by mutations in TREX1.46 It also encompasses cerebroretinal vasculopathy, hereditary endotheliopathy, retinopathy, nephropathy and stroke and hereditary vascular retinopathy.46 Patients develop visual symptoms secondary to the retinal vasculopathy in their fourth or fifth decade, followed by neurological features such as ischaemic strokes, TIAs, migraine, cognitive impairment, seizures and psychiatric symptoms. There is a progressive decline to mortality about 5–10 years after onset of symptoms.47 MRI can show pseudotumours in the deep white matter of the cerebrum and cerebellum, which are surrounded by vasogenic edema.48 Another characteristic feature that has been seen in patients with RVCL is Raynaud’s syndrome, strengthening the inclination towards a vascular phenotype.49
Hereditary infantile hemiparesis, retinal arteriolar tortuosity and leukoencephalopathy encompasses a spectrum with both infantile and adult onset with both neurological and systemic features,43 and is caused by autosomal dominant mutations in the COLA4A1 gene, which encodes the type IV collagen alpha 1 chain.50 Phenotypes can vary widely, with some cases presenting in childhood with porencephaly, infantile hemiparesis and developmental delay, and can develop intracranial cerebral hemorrhage (ICH) associated with trauma. Adults present most often with subcortical ICH.51 Neuroimaging demonstrates fluid-filled periventricular cysts that involve subcortical brain structures and intracranial aneurysms in intracranial portions of internal carotid artery. There can be white matter hyperintensities in supratentorial posterior periventricular, frontal and parietal areas. Arcuate fibres and temporal lobes are spared.51 Histopathology shows thickening and focal disruptions of capillary basement membranes.52
Mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes combines features of both migraine and stroke38 and is associated with MT-TL1 gene mutation. It is a multisystem disease, with age at onset in childhood with generalised tonic-clonic seizures and migraine-like episodes with headache, vegetative symptoms and abdominal complaints and subsequent recurrent episodes with acute neurological deficits. Sensorineuronal hearing loss is common. Other diagnostics include ragged red fibres on muscle biopsy, elevated lactate in cerebrospinal fluid and peripheral blood, and calcifications in the basal ganglia.38
Neuroimaging
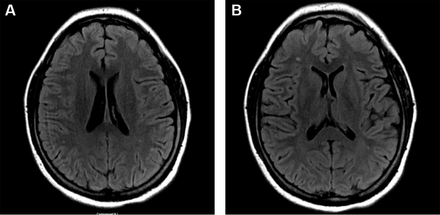
White matter hyperintensities (WMH) or white matter lesions are a common finding in patients with migraines, in addition to an increased prevalence of subclinical brain infarctions53 54 WMHs were visible as hyperintense lesions on T2-weighted and fluid-attenuated inversion recovery images (figure 2A,B) and as isointense or slightly hypointense on T1-weighted images. They are typically multiple, small, punctate hypertensities in deep or periventricular white matter and often seen on T2-weighted or images. Many studies have demonstrated the association between migraine and white matter changes, subclinical infarct-like lesions and volumetric change in grey and white matter on brain MRI.15 53 55–57 The connection is more robust in MA and is likely directly associated with chronic long-standing migraine and frequency of attacks. The clinical significance of white matter changes seen in migraineurs is still unclear. Some authors suggest that white matter abnormalities are due to ischaemic insults, which leads to the proposition that migraine could be a progressive brain disorder.58 A study from Copenhagen published last year investigated whether patients with MA have a higher risk for white matter lesions and silent strokes; it failed to identify a higher risk for silent strokes or white matter lesions in patients with migraine.59 The characteristics of WMHs can be rated by location, number and size. According to the Wahlund white matter lesions classifications,60 the higher classifications are linked with the degree of migraine. Lesions are mainly distributed in the frontal lobe, limbic system and parietal lobe.61 These white matter changes might be a useful marker for subsequent risk of stroke. Migraine treatments such as vasoconstrictors may be responsible for increase in white matter abnormalities in patients taking ergotamine whereas no increase in vascular changes are found from triptan use.24 There was a report that overuse of ergotamine may increase the risk of cerebral and cardiovascular disease62 63 whereas a link between the increased risk of stroke and triptans has not been identified.64 65 Although all the mechanisms underlying the increased prevalence of WMH in migraine are not yet well understood, they certainly deserve more attention and further investigation.
Typical manifestation of white matter change on MRI fluid-attenuated inversion recovery imaging in a 37-year-old female patient with chronic migraine.
Two meta-analyses of studies assessing WMH prevalence in migraine have shown that subjects with migraine are two to four times more likely to have WMHs than control subjects.15 66 The data presented by Swartz shows that there is a strong relationship between migraine and MRI white matter changes, regardless of comorbidities.66 The reason for this correlation has been attributed to alterations in resting cerebral blood flow (CBF), which can be interpreted by the change of certain metabolites. CBF is significantly lower in migraine with aura subjects with high white matter hyperintensity load.67 In a prospective study, Erdelyi-Botor and colleagues68 measured the serum L-arginine, asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA) in migraine patients in a headache-free period. The results demonstrate that patients with migraine with WMHs showed higher ADMA concentrations than lesion-free patients and controls; SDMA serum levels of lesional migraineurs were higher than in non-lesional patients. They concluded that elevated ADMA levels may impact the pathogenesis of migraine-related WMHs by influencing cerebrovascular autoregulation and vasomotor reactivity. In addition, higher SDMA concentrations may indirectly influence NO synthesis by reducing substrate availability, and elevated L-arginine serum levels might reflect an increased demand for NO synthesis. In juvenile patients with migraine, while migraine was a risk factor of WMHs, its relationship with arteriosclerotic factors was weak.69
Clinical management
Treatment options for patients with migraine should be tailored based on age, gender, risk factors and drug interactions. Antihypertensive agents including beta blockers, angiotensin II receptor blockers and ACE inhibitors (eg, lisinopril, olmesartan, candesartan) have shown a better effect than placebo in reducing frequency, severity and disability of migraine.70 Statin and statin with vitamin D have been reported to be efficacious for migraine prophylaxis in case reports.71–73 Although currently there is no recommended guideline for primary prevention of ischaemic stroke in migraineurs, if clinically indicated, it would be appropriate to select medications that reduce both migraine attacks and vascular risks in migraineurs. One of the most recent and important epidemiologic studies in 2016 was by Kurth and colleagues from the Nurses’ health Study 2, which studied 1 15 541 women (age range 25–42 years) who were followed for 20 years. In participating nurses who suffered from migraine, the HR was 1.5 for a major vascular event and 1.62 for stroke. It again showed there is a small absolute increase in the risk for stroke in female patients with migraine. Thus, it is extremely important to identify and modify vascular risk factors in this population including high blood pressure, smoking, oral contraceptives use and lifestyle changes.8
New drug delivery systems, such as mucoadhesive buccal discs and sublingual films with combinations of different medications, may achieve a rapid relief by fast onset of action and increased bioavailability, and may also reduce the side effects related to oral and parental treatment.74 Gavini et al reported that nasal administration of anti-migraine drugs through polymeric microcarriers could promote the central uptake of medication compared with oral or intravenous therapy, which potentially could decrease the dose needed to realise the same therapeutic effect by other administration routes.75 Another promising field is early diagnosis of endothelial dysfunction, which could help physicians to detect intracranial vascular changes before they can make a substantial impact on brain function. By combining conventional MRI with molecular MRI targeting P-selectin, Quenault et al were able to identify activated endothelial cells after an experimental TIA attack. This allows for discriminating transient ischaemic attack from epilepsy and/or migraine.76 In addition, due to increasing understanding about the diversity of human genotype and phenotype, and the decreasing cost of human genome sequencing, one may eventually be able to adjust the dose, type or combination of medications to make an individualised treatment plan for patients (based on race, age, gender and response to the therapy). The deepening appreciation of this disease and technological advances will eventually provide better care for our patients.
Conclusion
Migraine is associated with increased risk for stroke10 57 58 although aetiology of stroke in migraineurs remains unclear. It is imperative to raise more awareness and recognition of migraine as a risk factor for stroke and the close association between them. Future studies are needed to determine whether more aggressive prevention of migraine will decrease occurrences of migraine-related stroke and white matter lesions, moreover, whether such prevention demonstrates further clinical benefits. Early identification of risk factors for both conditions among patients may potentially decrease neurological morbidities.
References
Footnotes
Contributors YZ conceived this review and critically edited the manuscript for important content; AP and SQ helped draft and revise the manuscript.
Competing interests None declared.
Patient consent Not applicable.
Provenance and peer review Commissioned; externally peer reviewed.
Data sharing statement No additional data are available.