Article Text
Abstract
Background Hyperperfusion syndrome (HPS) is a rare but potentially a life-threatening complication after carotid artery angioplasty and stenting (CAS). Staged CAS has been an alternative to prevent HPS.
Materials and methods 44 of 908 patients with high-grade internal carotid artery stenosis or near occlusion were at risk of HPS because of poor collateral flow and impaired cerebral blood flow (CBF). They were treated with first (stage 1), followed by a full CAS (stage 2) 1 month later. Their 30-day outcomes were tabulated and analysed.
Results During follow-up, 1 of the 44 (2.2%) patients developed HPS immediately, 3 (7%) had postprocedural HPS (ie, transcranial Doppler (TCD) >120%) without clinical symptoms and 3 (7%) required stenting at stage 1 for carotid dissections. After stage 1, there were significant improvement between the preprocedural and postprocedural CBF (0.98±0.06 vs 0.85±0.05, p<0.05), mean transit time (MTT; 1.05±0.05 vs 1.15±0.05, p<0.05), time to peak (TTP; 1.04±0.06 vs 1.20±0.06, p<0.05) on CT perfusion (CTP), and CBF (66.41±7.41 vs 44.44±6.43, p<0.05) on TCD. After stage 2, improvement was seen in CBF (1.01±0.07 vs 0.98±0.06, p<0.05), MTT (1.01±0.05 vs 1.05±0.05, p<0.05), TTP (0.99±0.06 vs 1.04±0.06, p<0.05) on CTP and CBF (66.41±7.41 vs 93.78±18.81, p<0.05) on TCD. 2 had postoperative increase of middle cerebral artery mean flow velocity of 120% after stage 2 without clinical symptoms.
Conclusion Staged carotid artery stenting probably decreased the chance of developing HPS in this group of selected patients. Although requiring a 2-step intervention, staged CAS may be a safe and effective alternative.
- Carotid artery stenosis
- Cerebral blood flow
- Hyperperfusion
- Percutaneous transluminal angioplasty
- Stenting
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
- Carotid artery stenosis
- Cerebral blood flow
- Hyperperfusion
- Percutaneous transluminal angioplasty
- Stenting
Introduction
Carotid artery angioplasty and stenting (CAS) is a popular alternative to carotid endarterectomy to treat patients with carotid diseases. Hyperperfusion syndrome (HPS) is a rare but potentially a life-threatening complication after CAS with an overall incidence of 0.5–6.8%.1–3 The presence of internal carotid artery (ICA) stenosis ≥90% is a main risk factor for the development of HPS.4–7 Other important risk factors include severe contralateral ICA disease, poor collateral flow, hypertension, and recent stroke or ischaemia.8 The higher the number of risk factors present, the higher is the incidence of HPS, which ranges from 14.1% to 56%.8–13 How to prevent HPS remains controversial and there are no proven effective alternatives other than strict blood pressure (BP) control.1 ,14 However, it has been recently proposed that staged CAS by allowing gradual restoration of cerebral blood flow (CBF) would minimise HPS occurrence.14 We therefore have conducted a retrospective review of patients who were treated with the two-stage CAS at our centre and examined their outcome.
Materials and methods
Between October 2011 and June 2015, 908 patients with high-grade ICA stenosis had CAS. In the literature, the following criteria were used to select patients for the two-stage CAS:15 44 patients were identified to be eligible and included in this analysis. Their baseline medical condition and the presenting neurological events of each patient were recorded. Preoperative workup included brain MRI, helical CT scan, transcranial Doppler (TCD) scan of the cervical ICA and ipsilateral middle cerebral artery (MCA), digital subtraction angiography (DSA) to assess the collateral circulation status using the five-point scale proposed by ASITN/SIR,16 and CT perfusion (CTP) to assess CBF. The profile of each case has been summarised in table 1. All patients had signed the informed consent before the treatment and were included in this study.
Baseline characteristics of the 44 patients
We developed the following inclusion criteria for our series: (1) patient with symptomatic carotid stenosis (defined as stroke/transient ischaemic attack of the patient within 60 days who is taking antithrombotic drugs or under other interventions on vascular risk factors); (2) DSA showed carotid stenosis ≥90%, or near occlusion according to NASCET method; (3) poor collateral flow on DSA with a grade of ≤2 base on ASITN/SIR; and (4) cerebral hypoperfusion in the vascular territory of the culprit vessel on cerebral CTP imaging before procedure.
Using TCD to assess for HPS
Relative CBF changes were assessed by using TCD recordings of the flow velocity of the MCA 1 day before the procedure. During this period, the patient's systolic BP was maintained at >140 mm Hg. HPS is defined as an increase in CBF of >120% over baseline on TCD. Three patients had no temporal window. Finally 41 patients completed TCD scan.
Cerebral CTP
All patients received CTP study 1 day presatge and poststage 1 procedure and 1 day after stage 2 procedure. Asymmetry index (%) was calculated based on the measurement of blood flow between the two cortical hemispheres by taking the ratio of CBF of the affected to unaffected hemisphere excluding any ischaemic/infarcted areas (rCBF in the affected MCA territory/rCBF in the mirror position)×100. The outcomes are shown in table 2. Imaging information before procedure from a representative case is shown in figure 1.
The CTP parameters of patients with carotid artery stenosis regarding as high risk of hyperperfusion pre-pro procedure (n=41)
Images obtained of a 56-year-old man who manifested with transient right hemiparesis due to cerebral hypoperfusion caused by near occlusion carotid artery. (A) CT angiography showing near occlusion at the ICA origin; (B) right carotid angiography before angioplasty showing near occlusion at the ICA origin; (C) CTP scan in the resting state showing that CBF is severely decreased, CBV, MTT and TTP in the right cerebral hemisphere are severely increased. CTP, CT perfusion; CBF, cerebral blood flow; CBV, cerebral blood volume; ICA, internal carotid artery; MTT, mean transit time; TTP, time to peak.
Perioperative antiplatelet therapy
In order to minimise the risk of thromboembolic complications, regardless if a patient had asymptomatic or symptomatic carotid stenosis, all were given aspirin (100 mg/day) and clopidogrel (75 mg/day) at least 2 and 5 days prior to the procedure, respectively. Antiplatelet therapy was continued for 3 months after the stenting, and then all were on aspirin (100 mg/day) for life.
Stage 1: conventional balloon angioplasty
Semicompliant balloon (MONORAIL; Boston Scientific) was placed across the stenosis and inflated to a nominal pressure for 20 s (the diameter at full dilation was usually 2–3 mm). When the minimum luminal diameter exceeded 2.0 mm on angiography, the procedure was considered successful and completed. All patients had tolerated the procedure well. Imaging analysis during the procedure of stage 1 for the representative case is shown in figure 2.
Images obtained in a 56-year-old man who manifested with transient right hemiparesis due to cerebral hypoperfusion caused by near occlusion of the carotid artery. (A) Fluoroscopy during angioplasty showing that the target ICA stenosis was dilated by the balloon (diameter 2 mm, 8 atm for 30 s); (B) carotid angiography showing that the stenosis improved from >99% to <70% after angioplasty. (C) CTP scans in the resting state showing that CBF, CBV, MTT and TTP in the right cerebral hemisphere are significantly improved, after angioplasty. CTP, CT perfusion; CBF, cerebral blood flow; CBV, cerebral blood volume; ICA, internal carotid artery; MTT, mean transit time; TTP, time to peak.
Stage 2: CAS
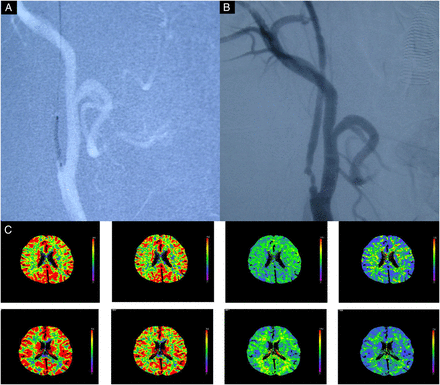
In this procedure, performed about 1 month after stage 1, an embolic protection device (Accunet, Abbott Laboratories) was used. While the distal ICA was protected with this system, the stenosis was predilated with a semicompliant balloon (Amiia or SAVVY; Cordis), followed by the deployment of a self-expanding stent (Acculink, Abbott Laboratories). When necessary, additional postdilation was performed in the same manner. A representative case is shown in figure 3. Information on imaging after stage 2 is shown in figure 3.
Images obtained from a 56-year-old man who manifested with transient right hemiparesis due to cerebral hypoperfusion caused by near occlusion of carotid artery. (A) Carotid angiography performed just before the stage 2 procedure shows remaining stenosis of the ICA, but there is no delayed filling of the distal part of the artery. (B) Carotid angiography performed after the procedure shows that the ICA stenosis is completely dilated after balloon angioplasty and stent placement. (C) After stage 2 angioplasty shows further CBF improvement in the right cerebral hemisphere, but not hyperperfusion. CBF, cerebral blood flow; ICA, internal carotid artery.
Postoperative BP management
All patients treated with regular CAS or SAP underwent continuous BP monitoring for at least 72 hours after the procedure. The goal was to control BP at a level lower than their preprocedural baseline value and maintain it for at least 1 month. All patients' antihypertensive treatment was followed up by telephone once a week once discharged home. There was no difference in the use of BP control protocol between stages 1 and 2.
Definition of HP phenomenon and HPS
Hyperperfusion (HP) phenomenon was defined as TCD detected increase in CBF of >100% over baseline or an asymmetry index of more than 120% in CBF compared with the normal side on CTP. HPS was used to designate the development of clinical symptoms as a result of rapidly increasing CBF in excess of that required to meet metabolic demands.2 After the operation, all patients were observed for any new neurological symptoms. Occurrence of HPS was suspected by one of the following clinical findings: throbbing frontotemporal or periorbital headache, confusion, macular oedema, visual disturbances, seizures or focal neurological deficits. If a patient had one of these symptoms or signs, cranial CT, CT angiography and cerebral perfusion CT would be performed immediately.
Statistical analysis
Statistical analysis was performed by using SPSS, V.21. All results were presented as means±SD. The significance of differences was determined by paired samples t-test. A p value <0.05 was considered significant.
Results
Between October 2011 and June 2015, 908 patients with high-grade ICA stenosis had CAS. Among them 44 patients qualified for this analysis. Demographics, risk factors and type of carotid stenosis are listed in table 1
There was no morbidity or mortality in all 44 patients who were treated by staged carotid artery stenting. Three of the 44 patients required direct stent placement because of dissection after the stage 1 treatment. One patient experienced HPS without morbidity, the second patient had HP phenomenon, the third patient was normal. After the stage 1 treatment, the hypoperfusion condition of the culprit vascular territory in the other 41 patients resolved significantly as seen on CTP and TCD, and the clinical symptoms of these patients also resolved after stage 2 treatment. One patient had a postoperative increase of MCA flow velocity of 120% after stage 2, but without clinical symptoms.
Balloon angioplasty and stent placement increased the CBF and decrease the MTT and TTP in patients with high-grade ICA stenosis or near occlusion
As shown in table 2, compared with preprocedure measurement, balloon angioplasty treatment produced a significant difference in CBF (t=27.35, p<0.05), mean transit time (MTT; t=19.16, p<0.05) and time to peak (TTP; t=25.63, p<0.05) on CTP. Meanwhile, there were also significant changes between the stages 1 and 2 of CBF (t=6.14, p<0.05), MTT (t=10.19, p<0.05) and TTP (t=6.84, p<0.05) on CTP.
CBV of balloon angioplasty and stent placement were similar to pre-procedure on CTP
As shown in table 2, Paired-Samples t-test showed that balloon angioplasty (t=0.70, p>0.05) and stent placement (t=0.854, p<0.05) did not produce any significant change in cerebral blood volume (CBV) on CTP after the procedure.
Balloon angioplasty and stent placement increased the CBF on TCD after the procedure
The effect of balloon angioplasty and CAS on CBF changes were assessed by TCD 1 day preprocedure and postprocedure (table 3) paired samples t-test showed that balloon angioplasty produced significant changes in CBF after stage 1 (t=31.06, p<0.05), and there was also significant difference of CBF between the stages 1 and 2 (t=13.44, p<0.05).
The TCD of patients with carotid artery stenosis regarded as having high risk of hyperperfusion preprocedure (n=41)
Discussion
Two-stage angioplasty was performed in patients with severely impaired CBF due to ICA stenosis who were judged to be at high risk of HPS. Conventional angioplasty without stent placement (stage 1) was successful in 41 patients in our institute. There was no evidence of HP phenomenon on postoperative TCD, and none of the patients manifested clinical symptoms attributable to the procedure. Only one patient had HP on postprocedural TCD during stage 2 procedure, but no clinical worsening or HP on CTP. These results suggest that HPS could be avoided in patients subjected to the two-stage carotid artery stenting in our consecutive cases, which was confirmatory to the finding by Yoshimura et al.12
The use of small-size (usually 2 mm in diameter) balloon catheters seemed helpful and safer during the stage 1 procedure. In our cases, a smaller balloon (2 mm) was enough to open the residual lumen and reduce cerebral hypoperfusion, which was seen on TCD and CTP. The symptoms of all patients were resolved after stage 1 procedure. It was unclear how long the residual lumen stayed open. If the lesion was dilated by using a larger size balloon catheter, a wall dissection could occur in the carotid artery. More studies are needed in order to determine the appropriate balloon size to safely open up the residual lumen.
High BP is known to be a significant risk factor for HPS after carotid intervention.5 ,12 Elevation of BP may increase cerebral perfusion pressure and predispose to ICH during reperfusion after carotid recanalisation. Postoperative BPs should be maintained to normal or slightly lower than normal values.17 ,18 However, HPS may also occur in normotensive patients or in participants with systolic pressure of <160 mm Hg, reflecting the role of impaired autoregulatory mechanisms.19–21 In our cases, the systolic BP in all of the patients was kept slightly lower than the baseline, but still there were three patients who experienced HP phenomenon on TCD, even after the systolic pressure was controlled to 20% lower than the baseline.
The pathophysiology of the hyperperfusion is multifactorial, while cerebral haemodynamics and cerebral autoregulation, as previously mentioned, are individualised in each patient. The most important preoperative risk factor of developing HPS is the high-grade ipsilateral carotid stenosis plus poor collateral flow. Patients with critical ICA stenosis often present with low cerebrovascular reactivity, which consists of maximal vasodilatation of cerebral arterioles in order to maintain sufficient cerebral blood perfusion.22 After CAS, the expanded lumen size increased CBF and led to hypertension. In contrast, autoregulation mechanisms were diminished and thus caused HP in the previously hypoperfused tissue. One critical factor is that the degree of ICA stenosis was ≥90%.4–7 In those who developed ICH, the mean ICA stenosis was 95%. In this group, the incidence of ICH was 3.8%. Lacking of interhemispheric collateral blood supply via the circle of Willis may be a marker for greater risk of developing reperfusion syndrome.3 Chang et al24 reported that prestenting CBV index >0.15 and TTP index >0.22with CTP were two independent parameters that might be associated with HPS after CAS.23 Yoshimura et al12 reported that 67% of patients who underwent regular one-stage CAS had HPS after stenting, but in the two-staged angioplasty group, none of the patients showed HPS. Based on this report, we also developed the criteria for the staged CAS for our patients in order to avoid HPS. For a more accurate haemodynamic assessment, the same protocol was used for CBF measurement with TCD and CTP in all patients before and after the treatment in this study. Stage 1 procedure helped to re-establish blood flow, which might restore the impaired cerebral microvascular autoregulation during the first month. During this period, the recovered cerebral vasoreactivity could control the constriction of small cerebral artery, and dampen the pressure from blood flow during the stage 2 procedure. That was likely the explanation why there was no patient who experienced HP symptoms and signs of CHS.
Although this treatment was successful in the current series, there were some limitations. First, the selection of the patients could be biased since the current study only focused on patients with severely impaired CBF and CVR. Second, other factors are also known to be risk factors for HPS after CAS: severe ipsilateral stenosis, isolated hemisphere with poorly developed communicating arteries, hypertension, and use of antiplatelet or anticoagulant therapy. Third, we did not introduce CVR in this study, which might impact the power of result of this study. Instead, CTP was commonly used rather than xenon-CT, single photon emissionCT or positron emission tomography. Nevertheless, when CBF decreased, combining the findings of MTT prolongation and decreased CBV could help confirm the dilation of the cerebral blood vessels; therefore, indicating that cerebral autoregulation has been impaired.24
Conclusion
In patients with impaired CBF from high-grade ipsilateral ICA stenosis, two-stage CAS treatment of the stenosis could reduce the risk of developing HPS. More studies are still needed to confirm the benefit. Nevertheless, it is relatively a simple operation with the two-stage CAS in patients with high-grade ICA stenosis and the risk of developing procedure-related complication is low.
References
Footnotes
DM and GL contributed equally.
Competing interests None declared.
Patient consent Obtained.
Provenance and peer review Commissioned; externally peer reviewed.
Data sharing statement No additional data are available.