Article Text
Abstract
Background Vascular cognitive impairment (VCI) is the second-leading cause of dementia worldwide, which is caused by cerebrovascular diseases or relevant risk factors. However, there are no appropriate animal models, which can be used to study changes of neuropathology in the human VCI. To better understand the development of VCI, we modified three mouse models of chronical vascular diseases, and further compared the advantage and disadvantage of these models. We hope to establish a more suitable mouse model mimicking VCI in human beings.
Methods Adult male C57/BL6 mice (n=98) were used and animals underwent transient bilateral common carotid arteries occlusion (tBCCAO), or bilateral common carotid artery stenosis (BCAS), or right unilateral common carotid artery occlusion, respectively. Haemodynamic changes of surface cerebral blood flow (CBF) were examined up to 4 weeks. Spatial cognitive impairment was evaluated to determine the consequence of chronic cerebral ischaemia.
Results These mouse models showed different extents of CBF reduction and spatial reference memory impairment from 1 week up to 4 weeks postoperation compared with the control group (p<0.05). We found that (1) bilaterally ligation of common carotid artery caused decrease of 90% CBF in C57/BL6 mice (p<0.05) and caused acute instead of prolonged impairment of spatial reference memory (p<0.05); (2) unilateral ligation of common carotid artery did not cause severe ipsilateral ischaemia as seen in the tBCCAO mice and caused minor but significant spatial reference memory disturbance (p<0.05); and (3) 20% decrease in the bilateral CBF did not cause spatial reference memory impairment 4 weeks postoperation (p>0.05), while 30% decrease in bilateral or unilateral CBF led to significant memory disturbance in mice (p<0.05).
Conclusion We demonstrated that BCAS using 0.16/0.18 mm microcoils is an alternative VCI mouse model when studying the mechanism and developing therapy of VCI.
- Brain
- Cerebrovascular Disorders
- Cognitive Dysfunction
Data availability statement
Data are available in a public, open access repository.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
Cerebral ischaemia or hypoperfusion leads to cognitive impairment in experimental mouse models.
WHAT THIS STUDY ADDS
This study elucidates different cerebral ischaemia and spatial cognitive impairment patterns in three common vascular cognitive impairment (VCI) mouse models.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
Researchers can use 0.16/0.18 mm bilateral common carotid artery stenosis as an alternative VCI mouse model when they study the mechanism and the development of VCI therapy.
Introduction
Vascular cognitive impairment (VCI) represents the second-leading cause of dementia after Alzheimer’s disease, accounting for 15%–40% of all dementia cases.1 2 The development of VCI is a complex consequence of various cardiovascular and cerebrovascular events, such as cerebral small vessel disease, multi-infarct dementia, strategic infarct, severe hypoperfusion, hereditary vasculopathy and other haemorrhagic cerebrovascular diseases.3 4 In VCI patients, the main cerebrovascular pathologies are usually cortical and subcortical micro, multiple infarcts and white matter hyperintensities due to chronic or/and acute compromised cerebral blood flow (CBF) in the whole brain or specific brain regions. The other neuropathophysiological changes include: brain–blood barrier breakdown, myelin loss, arteriolosclerosis, leptomeningeal cerebral amyloid angiopathy and perivascular space dilation, etc.5 6 Although much has known about VCI, the precise mechanism that how these neurovascular pathologies translate to cognitive function decline remains unrevealed.
Currently, more than 16 rodents experimental models have been developed to mimic neuropathological features and symptoms of human VCI.7 Usually, most experimental VCI models depended on generating chronic cerebral hypoperfusion (CCH) condition, resulting in white matter injury, neuronal loss, axonal damage and cognitive impairment in animals.7 8 Apart from CCH, some studies found transient cerebral hypoperfusion could also cause acute or remote neurological damage and subsequently resulted in cognitive impairment.9 Although CCH is a key mechanism leading to VCI and dementia, none of CCH models could replicate all neuropathological abnormalities in VCI patients because simply blocking or narrowing major cervical artery could not completely cause real cerebral ischaemia pattern occurring in VCI patients.10 Therefore, it is necessary to know the different characteristic of these experimental animal models for selecting an better model for experimental studies and clinical translation of drugs and therapies.
Among all animals, rodent species, especially C57/BL6 mouse strain were preferred to be used to establish VCI model because of many advantages: such as less drug dosage, easier handling, and more importantly, higher vulnerability to cerebral ischaemia because of incomplete circle of Willis.11 Therefore, this study established three commonly used experimental mouse models of VCI using C57/BL6 mouse strain by ligation or stenosis of common carotid arteries (CCAs), namely transient bilateral common carotid arteries occlusion (tBCCAO), or bilateral common carotid artery stenosis (BCAS), or right unilateral common carotid artery occlusion (rUCCAO). For BCAS models, we set three subgroups according to different extents of CCA stenosis caused by microcoils with different inner diameters (ID, eg, bilateral 0.16 mm, 0.16/0.18 mm and bilateral 0.18 mm). We aimed to clarify which mouse model is more appropriate to induce chronic brain ischaemia and closely mimicking the CBF changes of VCI patients, providing a valuable scientific research tool to elucidate the pathophysiology and develop therapeutic strategy for VCI in human.
Materials and methods
Animal experimental design
Animal studies were reported following ARRIVE guidelines. Adult male C57/BL6 mice (8 weeks, weight 22–24 g) were provided by Beijing Vital River Laboratory Animal Technology. Mice were acclimatised for 1 week before operation. Mice were housed at 22 °C with 60% humidity and were provided ad libitum access to food and water. For CBF analysis, each experimental model’s CBF was compared with the preoperation baseline, so no control group was included. For cognitive function analysis, each model was compared with sham-operation. The experimental unit was single mouse. A total number of 118 mice were used for this study, 20 mice failed to survive from the operation because of severe cerebral ischaemia. The remaining 98 mice were further used for the whole experiment.
Bilateral common carotid artery stenosis
The BCAS model was established by using microcoils specifically designed for the mouse (microcoil specifications: piano wire diameter: 0.08 mm, total length: 2.5 mm, internal diameters: 0.16 mm and 0.18 mm, Anruike Biotechnology, Xi 'an, China). Animals (n=56) were induced anaesthesia with 5% isoflurane/95% air mixture and maintained to anaesthesia by 5% isoflurane/95% air mixture during operation. The left and right CCA were exposed and isolated from the surrounding sheaths. Then microcoils were twined by rotating it around each CCA. The site of operation was sutured. During the operation, 16 mice failed to survive because of the acute severe CBF reduction. The survived mice were observed and taken care of post-operation until being conscious and recovered to freely access food and water ad libitum. The operated mice were further divided into three subgroups according to different internal diameters of the microcoils: 0.16 mm group (n=7), 0.16/0.18 mm (0.16 mm for the right CCA/0.18 mm for the left CCA) group (n=10) and 0.18 mm group (n=11). Mice in the control group underwent the identical operation without occlusion of CCAs (n=12).
tBCCAO and rUCCAO
Transient bilateral common carotid artery occlusion
Anesthetisation procedure was same as the BCAS model. The right and left CCAs were isolated from the adjacent nerves and surrounding sheaths, and both CCAs were occluded with microvascular clips (RWD life sciences, Shenzhen, China) for 20 min. Then the clips were removed to allow CBF reperfusion and incisions were sutured. Mice in the control group were sham-operated for 20 min without occlusion of CCAs. After the operation, mice were kept in their quarters with food and water available ad libitum.
Right unilateral common carotid artery occlusion
Mice were administered and fixed on the operation table on supine position. The right CCA was isolated from the adjacent nerves and ligated with a 6–0 silk suture to achieve rUCCAO. A total number of 62 mice were used for the operation, in which 4 mice died during the operation because of severe cerebral ischaemia, the survived mice were used for the following experiments (tBCCAO group, n=33; rUCCAO group, n=12; control group, n=13).
Surface CBF measurement
The surface CBF of each time point of animal models were measured by laser Doppler flowmetry (Moor Instruments, Devon, UK). The measurement method of the surface CBF was described as our previous study.12 Briefly, mice were anaesthetised with isoflurane and their scalp was cut to expose the skull. The detector was then placed on the skull 1 mm posterior and 2 mm lateral to the bregma and held for a few seconds until the floating CBF value was stabilised. Then, the CBF value was recorded, and the scalp was sutured.
Laser speckle contrast imaging
Laser speckle contrast imaging (LSCI) measurement method was described as our previous study.13 Briefly, mice were anaesthetised and heads were fixed in a stereotaxic frame. Then mice were placed under the LSCI equipment (RFLSI Pro, RWD life sciences, Shenzhen, China). The scalp of mice were cut by surgical scissors and the surface of the skulls were exposed and illuminated with a 784 nm/32 mW laser with a beam expander and light intensity was controlled by a polariser. The CBF images were detected by a CCD camera and the image acquisitions were performed using custom software (RWD life science). Three hundred frames were acquired at 10 Hz with 10 ms exposure time.
Morris water maze test
The Morris water maze (MWM) protocol in this study was followed by Vorhees et al.14 Mice were subjected to MWM test for spatial learning and memory evaluation. A circular pool (diameter: 20 cm; height: 50 cm) filled with water at 22 °C was divided into four equal quadrants. A video camera was hanging over the pool and four different references were hung on the wall of different directions. A white platform (diameter: 8 cm) was submerged 1 cm below the surface of the water surface. The position of each reference inside and outside the maze was kept unchanged during the training sessions. For the acquisition period, each mouse received one session per day for 5 days (D1–D5) consecutively. Each session consisted of four trials with an interval of 10 min. During each trial, mice were gently placed in the pool towards the wall of the maze at one of the four starting positions. The time mice spending on finding the hidden platform (escape latency) and the swimming track were automatically recorded by the Anymaze software (Stoelting, Naperville, USA). The maximum test time for each per is 60 s. If the mouse failed to locate the target platform within 60 s, then it was guided to the platform and stayed for 15 s. On D6, the platform was removed, mice were subjected to a probe test. The time and distance mice spending in the target quadrant (quadrant concluding the platform) and times that mice crossed the platform area were all recorded by the Anymaze software.
Statistical analysis
All statistical analyses were performed using the GraphPad Prism V.8.0 (GraphPad Software, San Diego, USA). The normality of all data was analysed by D'Agostino and Pearson omnibus normality test. The significance of differences was determined by one-way analysis of variance, followed by multiple comparisons between the control group and experimental model groups. All values were presented as mean±SEM P<0.05 was consider significant difference. *Indicated p<0.05; **indicated p<0.01; ***indicated p<0.001.
Randomisation
No randomisation method was used in this study, animals were open-labelled according to the number registered in Anymaze software.
Inclusion and exclusion criteria of animals
The assays of CBF changes and cognitive impairment were exploratory in this study, so no inclusion and exclusion criteria were used.
Results
CBF changes in three cerebral hypoperfusion models
We first examined haemodynamic changes in BCAS mice. BCAS subgroups operation procedure was shown in figure 1A. The representation of CBF change detected by LSCI for each BCAS subgroup was shown in figure 1B. We found that the CBF in 0.16 mm group reduced to bilaterally 45%–50% of baseline postoperation, recovered to about 60% after 1 week and reached 70% of the baseline after 4 weeks (figure 1C). In mice of 0.16/0.18 mm group, the CBF of the right hemisphere (subject to ID=0.16 mm microcoil) declined to 45%–50% of baseline level postoperation and gradually recovered to 65%–70% after 4 weeks. The CBF of left hemisphere (subject to ID=0.18 mm microcoil) decreased to 55%–60% postoperation and gradually recovered to 70% 4 weeks later (figure 1D). In mice of 0.18 mm group, CBF decreased bilaterally to 60%–65% of baseline postoperation, and then gradually recovered to about 80% of baseline by the fourth week (figure 1E). The rUCCAO and tBCCAO operation procedures were shown in figure 2A. The representations of CBF change detected by LSCI for rUCCAO and tBCCAO mice were shown in figure 2B. In mice of rUCCAO group, the CBF was reduced to around 55% of baseline while the contralateral CBF reduced about 10%–15% compared with preoperation. One-week postoperation, ipsilateral CBF returned to 75%–80% and the contralateral CBF recovered to baseline level. Four weeks later, the ipsilateral CBF recovered to 80% of baseline level and contralateral CBF stayed nearly 100% (figure 2C). In mice of tBCCAO group, the bilateral CBF decreased to approximately 10% of baseline level and returned to 100% after releasing vascular clamps. The bilateral CBF showed no significant difference from the first to the fourth week (figure 2D).
CBF changes and mortalities of BCAS models (A) Schematic representation of BCAS operation (grouping by different inner diameters of the microcoils). (B) Representation of laser speckle contrast image on BCAS model subgroups at preoperation, 2 hours postoperation, and 1 week, 2 weeks, 4 weeks postoperation. (C–E) Numerical representation of CBF changes measured by laser Doppler flowmetry at preoperation, 2 hours postoperation, and 1 week, 2 weeks, 3 weeks and 4 weeks postoperation. (C) The 0.16 mm subgroup of the BCAS model. (D) The 0.16/0.18 mm subgroup of the BCAS model. (E) The 0.18 mm subgroup of the BCAS model. n=5/group. (F) The mortality of different subgroups. All data were represented as mean±SEM. ***P<0.001. BCAS, bilateral common carotid artery stenosis; CBF, cerebral blood flow.
Cerebral blood flow (CBF) changes and mortalities of right unilateral common carotid artery occlusion (rUCCAO) and transient bilateral common carotid arteries occlusion (tBCCAO) models (A) Schematic representation of tBCCAO and rUCCAO operation. (B) Representation of laser speckle contrast image of rUCCAO and tBCCAO models at preoperation, 2 hours postoperation (rUCCAO)/immediately postoperation (tBCCAO), and 1 week, 2 weeks, 4 weeks postoperation. (C–D) Numerical representation of CBF changes measured by laser Doppler flowmetry (LDF) at preoperation, 2 hours postoperation (rUCCAO)/immediately postoperation (tBCCAO), and 1 week, 2 weeks, 3 weeks and 4 weeks postoperation. n=5/group. (C) rUCCAO. (D) tBCCAO. (E) Mortalities of tBCCAO and rUCCAO operation. All data were represented as mean±SEM. ns indicated not significant.
Mortality
The mortality of 0.16 mm, 0.16/0.18 mm and 0.18 mm group in the BCAS model reached nearly 75%, 20% and around 15%, respectively (figure 1E). The mortality of rUCCAO and 20 min-tBCCAO models was both around 10% (figure 2E).
Spatial reference memory and study function
Bilateral common carotid artery stenosis
Four weeks’ postoperation, the spatial reference study and memory of the 0.18 mm group was not statistically different compared with the control group (figure 3A–D), while both 0.16 mm and 0.16/0.18 mm group showed significantly impairment of cognitive function as evidenced by a slower study rate to locate the platform (figure 3A) and less time spent in the target quadrant as well as less crosses to the platform area (figure 3B,C). Notably, mice in 0.18 mm and 0.16/0.18 mm groups showed no motor ability impairment after 4 weeks, while mice in 0.16 mm group showed motor ability impairment evaluated by the swimming speed (figure 3D).
Transient bilateral common carotid arteries occlusion
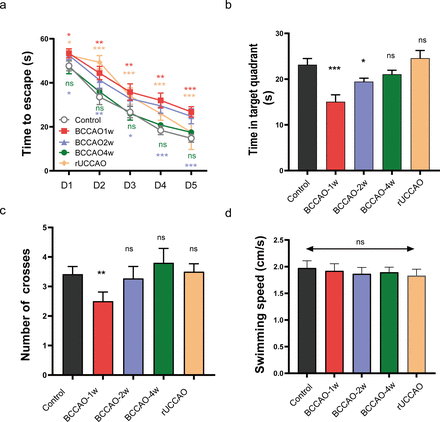
Mice underwent tBCCAO showed significant decline in spatial reference study function at first and second weeks’ postoperation. However, 4 weeks’ postoperation, the performance of tBCCAO mice in MWM was not significantly different from that of the control group (figure 4A–C). The tBCCAO mice did not show any impairment in motor ability in this experiment, as evidenced by no reduction in the swimming speed compared with the control group (figure 4D).
Test of spatial reference memory and motor ability deficiency of BCAS model subgroups (A) Morris water maze (MWM) acquisition period for subgroups of the BCAS model from the starting day 1 to day 5 of the test. (B) MWM probe test on day 6. (C) Times of each mouse swimming across the platform area in probe test. (D) Times of each mouse swimming across the platform area in probe test. (E) Average swimming speed of each group on day 6. All groups were compared with the control group. All data were represented as mean±SEM. ns indicated not significant; *p<0.05; **p<0.01; **p<0.001. 0.16 mm subgroup (n=7); 0.16/0.18 mm subgroup (n=10); 0.18 mm subgroup (n=11); control group (n=12). BCAS, bilateral common carotid artery stenosis.
Test of spatial reference memory and motor ability deficiency of right unilateral common carotid artery occlusion (rUCCAO) and transient bilateral common carotid arteries occlusion (tBCCAO) models. (A) Morris water maze (MWM) acquisition period for tBCCAO at 1 week, 2 weeks and 4 weeks after operation from day 1 to day 5. (B) MWM probe test on day 6. The time each mouse swimming in the quadrant including the platform area. (C) The time each mouse swimming across the platform area in probe test. (D) Average swimming speed of each group on day 6. All data were represented as mean±SEM. All groups were compared with the control group. NS indicated not significant; *p<0.05; **p<0.01; ***p<0.001. tBCCAO 1 week, 2 weeks, 4 weeks group (n=11/group); rUCCAO group (n=12); control group (n=13).
Right unilateral common carotid artery occlusion
The rUCCAO mice showed mixed results. In the acquisition period, mice showed a longer time to find the platform compared with that in the control group, while they behaved almost as good as the control group in the probe text (figure 4B,C). Similarly, rUCCAO mice showed no motor ability impairment (figure 4D).
Discussion
In this study, we compared three common mouse VCI models by acute or chronic restriction of CBF. Our results showed that: (1) Bilaterally ligation of CCAs caused 90% CBF decrease in C57/BL6 mice and caused acute instead of prolonged impairment of spatial reference memory; (2) Unilateral ligation of the CCA did not cause as severe ipsilateral ischaemia as seen in tBCCAO mice but caused significant spatial memory disturbance and (3) Narrowing the CCAs by half of its diameters (ID: 0.18 mm) caused approximately 20% decrease after collateral compensatory, but did not lead to spatial reference memory after 4 weeks’ postoperation detected by MWM paradigm. However, narrowing 75% of CCAs (ID: 0.16 mm) caused severe spatial cognitive and motor function impairment with a very high mortality rate in C57/BL6 mice; (4) Both mice in 0.16/0.18 mm and 0.18 mm groups caused significant spatial cognitive impairment with only mild mortality.
Mouse species was preferred by researchers in the field of cerebral ischaemia/hypoperfusion compared with rats due to its unique advantages, such as more vulnerability to cerebral ischaemia and easier gene modification.9 15 As mouse stain could not survive from the permanent BCCA ligation,11 temporary ligation of CCAs was a common procedure to construct VCI in mice in many studies.16 Studies using the tBCCAO model demonstrated that a 5–20 min ligation of bilateral whole-brain ischaemia caused obvious neuropath physiological changes, such as blood–brain barrier breakdown, white matter injury and early or late hippocampal neuronal damage,5 17 18 as well as decreased spatial memory and learning function.19 In our study, mice subject to bilateral transient whole-brain ischaemia showed about 90% reduction of CBF along with acute (up to 2 weeks’ postoperation) but restorable (restored after 4 weeks) spatial cognitive impairment. Some other studies also found a decline in spatial reference memory of tBCCAO and suggested that this decline was related to delayed damage of hippocampal neurons, especially in the CA1 area.18 19 Although the advantages of the tBCCAO model, it lacked major similitude of the real disease state because rare VCI patients were caused by single anterior-circulation ischaemia. Moreover, 20 min tBCCAO did not precisely mimic the humbler diminishments in CBF seen in CCH conditions related to human VCI caused by CCH where CBF might only be reduced by 20%–30%.20
The rUCCAO model was proposed by Japanese scholar Yoshizaki et al as an experimental mouse model of CCH.21 The rUCCAO model has numerous advantages compared with other models, such as more consistent CBF decrease among subjects due to easy operation, lower mortality and higher consistency within the same group. This study confirmed that the ipsilateral CBF dropped to 55% of baseline after ligation and gradually recovered to 80% after 4 weeks’ postoperation, which was much similar to that of mice in the 0.18 mm group of BCAS model. In addition, the impairment of spatial reference memory function in both of these two models was insignificant. However, other studies found that rUCCAO mice showed impaired cognitive function evaluated by novel object recognition and MWM tests.22 23 The difference between our study and others still is unknown and needs to be further investigated.
The mouse BCAS model was first established in 2004 with a better simulation of clinical BCCA stenosis without causing significant visual function impairment.24 Therefore, it was soon became the widely used model for the research of CCH, white matter injury and VCI.25 26
It is true that Ihara had explored the CBF reduction in BCAS mice induced by different diameter microcoils (0.16, 0.18, 0.20 and 0.22 mm).24 However, previous studies showed that the BCAS model induced by 0.18 mm diameter microcoils could not induce consistent CBF reduction,27 28 which limited its clinical translation. Currently, 0.16 mm and 0.18 mm diameter microcoils were most widely, while few 0.17 mm diameter microcoils were used to establish the BCAS mouse models.24 29–31 Therefore, we chose 0.16 mm and 0.18 mm diameter microcoils to induce the BCAS models in this study. Since mice underwent chronic brain ischaemia induced by 0.16 mm diameter microcoils showed a high mortality (75%),32 we further explored CBF reduction in BCAS mice induced by 0.16/0.18 mm diameter microcoils (using a 0.16 mm diameter microcoil on one side of the CCA and a 0.18 mm diameter microcoil on the other). We aimed to clarify whether 0.16/0.18 mm diameter microcoils achieved a balance between the mortality and severity and which diameter microcoil was more appropriate to induce the BCAS model closely mimicking the CBF changes of VCI patients. In addition, we compared the CBF reduction pattern in the BCAS model with the other common VCI mouse models including tBBCAO and rUCCAO. Lastly, the spatial reference memory impairment was investigated among these three VCI mouse models. We demonstrated that the BCAS model was more likely to decipher the haemodynamics changes in VCI patients, and the 0.16/0.18 mm diameter microcoil was more appropriate to induce the BCAS model in mice because of the relative low mortality and lasting CBF reduction. Moreover, the asymmetrical ischaemia of right and left hemispheres in this model was closer to the clinical situation in which patients with carotid plaque usually showed asymmetric stenosis of the carotid artery.20 33 Therefore, we assumed that the 0.16/0.18 mm BCAS model was an appropriate alternative for VCI study.
In this study, animal surgery was operated by the same experienced surgeon. All raw data were presented as mean±SEM. In addition, previous study reported similar mortality of mice with BCAS surgeries induced by 0.16 mm and 0.18 mm diameter microcoils, respectively.24 Thus, although it exists bigger differential mortality among 16 mm group (75%), 0.16/01.8 mm group (20%) and 0.18 mm group (15%), our results were truly reflecting the effect of different diameter microcoils on CBF reduction and brain damage.
MWM is the most widely employed behavioural test for assessing spatial cognitive function deficits. MWM is also an important behavioural paradigm for drug, molecular, and gene screening in bench studies because of its relatively good objectivity and consistency without causing injury to animals.34 However, this study only focused on deficits in spatially reference memory 4 weeks’ postoperation and lacked exploring other dimensions of cognitive function using more paradigms, such as spatial working memory or fear-based memory. Different cognitive paradigms usually reflected impairments in different brain regions. For example, spatial working memory mainly depended on integrity of white matter while spatial memory was more on decoding function of hippocampus.35 36 Second, we only detected cognitive impairment 4 weeks’ postoperation, which might not sufficiently estimate the impact of CCH on cognitive function since the emergence of cognitive impairment was a chronic process in real VCI patients. Some studies found that 0.18 mm BCAS mice showed impairment of motor function and spatial reference memory alone with some other pathological changes not shown within short period after 6 months.7 37 Therefore, it is critical to further evaluate the impact of compromise CBF on the neuropathy and cognitive impairment after a longer time postoperation.
In conclusion, our study provided valuable information for scientific researchers to choose 0.16/0.18 mm BCAS as an appropriate mouse model to elucidate the pathophysiology and develop therapeutic strategy for VCI in human.
Data availability statement
Data are available in a public, open access repository.
Ethics statements
Patient consent for publication
Ethics approval
All animal experiment procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC), Fudan University, Shanghai, China.
Acknowledgments
We are especially thankful to all the members of Yang Lab, Med-X Research Institute and School of Biomedical Engineering, Shanghai Jiao Tong University for the aid they offered in this study.
References
Footnotes
ZZ and YM contributed equally.
Correction notice This article has been amended since it was first published. There was a spelling mistake in figure 4 and this now been corrected.
Contributors ZZ collected data and wrote the manuscript; YM helped revise figures and the manuscript; TX and SW helped do the animal models and also the behavior test; G-YY helped revise the manuscript; JD and XW were responsible for the overall content as the guarantors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.