Article Text
Abstract
Objectives Incident ischaemic stroke (IS) risk may increase not only with lipids concentration but also with longer duration of exposure. This study aimed to investigate the impact of cumulative burden of lipid profiles on risk of incident IS.
Methods A total of 43 836 participants were enrolled who participated in four surveys during 2006–2013. Individual cumulative lipid burden was calculated as number of years (2006–2013) multiplied by the levels of low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), non-HDL-C and triglyceride (TG), respectively. The primary outcome was defined as the incident IS during 2012–2017.
Results During 4.67 years (±0.70 years) follow-up on average, we identified 1023 (2.33%) incident IS. Compared with respective reference groups, the HRs (95% CIs) of the upper tertile in cumulative TG burden, cumulative LDL-C burden, cumulative TC burden and cumulative non-HDL-C burden were 1.26 mmol/L (1.02–1.55 mmol/L), 1.47 mmol/L (1.25–1.73 mmol/L), 1.33 mmol/L (1.12–1.57 mmol/L) and 1.51 mmol/L (1.28–1.80 mmol/L) for incidence of IS, respectively. However, this association was not significant in cumulative HDL-C burden and IS (HR: 1.09; 95% CI: 0.79 to 1.52), after adjustment for confounding variables. Among 16 600 participants with low cumulative LDL-C burden, HRs (95% CI) for TC, TG, non-HDL-C and HDL-C with IS were 1.63 mmol/L (1.03–2.57 mmol/L), 1.65 mmol/L (1.19–2.31 mmol/L), 1.57 mmol/L (1.06–2.32 mmol/L) and 0.98 mmol/L (0.56–1.72 mmol/L), respectively.
Conclusions We observed the correlation between cumulative burden of lipid profiles, except for cumulative burden of HDL-C, with the risk of incident IS. Cumulative burden of TC, TG and non-HDL-C may still predict IS in patients with low cumulative LDL-C burden.
Trial registration number ChiCTR-TNRC-11001489.
- stroke
Data availability statement
Data are available upon reasonable request. Data are available to researchers on request for purpose of reproducing the results or replicating the procedure by directly contacting the corresponding author.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Hyperlipidaemia has been widely documented to be associated with higher cardiovascular disease and ischaemic stroke (IS) risk.1 2 Although lowering low-density lipoprotein cholesterol (LDL-C) levels is the primary target of therapy in most clinical guidelines,3–5 accumulating evidence indicates that other lipoprotein-lipid measurements could provide a predictive value over and above that of LDL-C levels.6 For example, individuals with low levels of high-density lipoprotein cholesterol (HDL-C) are also likely to experience cardiovascular events.7 8 Furthermore, individuals treated with statins who achieve low LDL-C levels, but have high concentrations of either non-HDL-C or apolipoprotein B (ApoB), remain at increased cardiovascular risk.9 10
More importantly, atherosclerosis is a slowly progressive disorder influenced by suboptimal lipid levels. Long-term versus contemporary lipid levels may more strongly impact the development of atherosclerosis disease.11 Ference et al described the cumulative effect of lipid-carrying lipoproteins on the risk of cardiovascular disease.12 Once the threshold of cumulative LDL-C has been exceeded, the risk of experiencing an acute coronary syndrome in response to continued plaque growth increases log linearly.13 Previous study proposed that the causal effect of LDL-C on the risk of cardiovascular disease is determined by both the magnitude and the cumulative duration of exposure to these lipoproteins.14 Additionally, the UK Biobank study demonstrated the association between long-term exposure to lower levels of LDL-C and with lower risks of cardiovascular events, in a dose-dependent way.15
Stroke remains the leading cause of disability and mortality worldwide.16 17 The primary prevention of stroke is of great importance. Few studies have examined the association of exposure duration of lipid levels with incident IS in general population. In this prospective study, we aimed to investigate the effect of cumulative burden of lipid parameters on subsequent IS in general population and its residual risk in participants maintaining an ideal LDL-C level.
Methods
Study population
The Kailuan study is a prospective cohort study designed to investigate the risk factors or common non-communicable diseases by collecting data from health examinations.12 18–20 The study design has been detailed previously. The Kailuan study prospectively enrolled 101 510 participants aged 18–98 years agreed to participate and completed the first survey from June 2006 to October 2007 (referred to as the 2006 survey here). Participants underwent questionnaire surveys, physical examinations and laboratory tests in 11 hospitals responsible for the healthcare of the community biennially in the four surveys (2006–2007, 2008–2009, 2010–2011 and 2012–2013) to calculate the cumulative burden of lipid profile. The current study is a pre-designed subanalysis of the Kailuan study. A diagram of the current study was presented in supplementary materials (online supplemental figure S1). Of these 101 510 participants, there were 47 832 participants who completed all the four surveys. After excluding 1399 participants with any stroke (IS or haemorrhagic stroke), myocardial infarction, cancer until last examination and 2594 participants with missing data on any of the study variables in the four surveys, 43 836 participants were included in the final analysis (figure 1).
Supplemental material
Flowchart of the study. HDL, high-density lipoprotein; LDL, low-density lipoprotein; TC, total cholesterol; TG, triglyceride.
This study was approved by the ethics committee of the Kailuan General Hospital. All subjects have signed informed consents. De-identified data were used for analyses.
Lipid measurements
Blood samples were obtained from the antecubital vein after an overnight fasting and transfused into vacuum tubes containing EDTA. Serum was separated immediately and stored at 4 °C. The analysis was conducted within 4 hours of blood sample collection using an auto-analyzer (Hitachi 747; Hitachi, Tokyo, Japan) at the central laboratory of the Kailuan Hospital. TC was measured using the endpoint test method. HDL-C and LDL-C levels were measured using a direct test method and TG were measured using the glycerol phosphate oxidase (GPO) method (inter-assay coefficient of variation: <10%; Mind Bioengineering Co, Shanghai, China). Non-HDL-C levels were determined by subtracting serum HDL-C levels from total cholesterol.21
Definition of cumulative lipid burden and scoring
We applied 5.2 mmol/L (200 mg/dL), 2.26 mmol/L(200 mg/dL),2.6 mmol/L (100 mg/dL), 1.0 mmol/L(40 mg/dL) and 3.4 mmol/L (130 mg/dL) as cut-off values of TC, TG, LDL-C, HDL-C and non-HDL-C respectively, for the calculation of cumulative burden of lipid profile.22 Cumulative burden of individual lipid or lipoprotein was calculated according to the equation below:
Cumulative burden=((value1+value2)/2–cut-off)×interval years1–2+((value2+value3)/2–cut-off)×interval years2–3+((value3+value4)/2–cut-off)×interval years3–4.23
When the average of value1+value2 was lower than its cut-off value, the corresponding cumulative burden between the two consecutive visits would be defined as 0. Participants who had cumulative burden of individual lipid or lipoprotein >0 were classified into tertile groups, and defined as a cumulative burden score of 1, 2 and 3, respectively.
Assessment of potential covariates
Information on age, sex, smoking, physical activity, alcohol drinking and medication histories (eg, hypoglycaemic, antihypertensive and lipid-lowering agents) was collected via a questionnaire survey as detailed previously.24 Weight and height were measured by trained nurses. Body mass index (BMI) was calculated as weight in kilogrammes divided by the square of height in metres.
Study outcome and follow-up
The primary outcome was the first occurrence of IS. Second outcomes included myocardial infarction and all-cause death. Ascertainment of incident IS was described previously.25 IS was defined according to ICD-10 (international Classification of diseases (ICD)) criteria (codes I63 or I64) based on characteristic signs, symptoms and finding of brain CT or MRI. Myocardial infarction was defined according to ICD-10 criteria (codes I21 or I22) based on a clinical history of typical cardiac symptoms, cardiac biomarkers and changes on serial electrocardiograms. Mortality data were obtained from the registration information on State Vital Statistics Office. To collect outcome data, the follow-up of the study population was continued from 1 January 2012 until the occurrence of any defined IS, or 31 December 2017, whichever came first.
Statistical analysis
Continuous variables were presented in medians (IQRs) and compared between groups using the non-parametric Wilcoxon test. Categorical variables were presented as percentages and tested by χ2 test. We used Cox proportional hazards models to estimate HRs and 95% CIs of the study outcome based on cumulative burden of lipid profile. We fit three multivariate models: model 1 adjusted for age and sex; model 2 further adjusted for physical activity (never, four times a week or ≥four times a week), smoking status (never, past or current smoker), alcohol status (never, past drinker, current drinker: 2, 2–4 or ≥5 servings per day) and smoking status (never, past or current smoker), at baseline (the survey of 2012–2013); and model 3 further adjusted for history of hypertension, diabetes mellitus, hypercholesterolaemia. Cumulative burden of lipid profiles were also treated as continuous variables in complementary analysis. Each cumulative burden of lipid profile was divided into four groups by quartiles. We also performed stratified analysis to test association between cumulative burden of lipid profiles and IS by one time point of LDL levels. To test the residual risk of IS, we additionally performed Cox proportional hazards models to estimate the association between the cumulative burden of other lipid profiles and incidence of IS in participants with cumulative LDL-C burden=0, which represented low cumulative LDL-C burden. The cumulative incidence of the outcomes by cumulative burden groups was calculated using the Kaplan-Meier approach.
Formal hypothesis testing was two-sided with a significance level of 0.05. All statistical analyses were conducted using SAS V.9.4.
Results
Study population
A total of 43 836 participants were enrolled in this study. We presented comparison between participants included and excluded (table 1). There were differences in age, sex, BMI, smoker, history of hypertension, diabetes mellitus, hypercholesterolaemia between participants included and excluded. During the follow-up period (from 2012 to 2017) of 4.67±0.70 years, we identified 1023 (2.33%) incident IS cases among 43 836 participants. Events number of myocardial infarction and all-cause death were 272 (0.62%) and 1343 (3.06%).
Baseline characteristics of participants included versus not included in this subanalysis of the Kailuan cohort
Individual cumulative burdens and risk of IS
After multivariable adjustment for confounding factors at baseline (the survey of 2012–2013), compared with the reference group, higher cumulative TG, LDL-C, TC and non-HDL-C burden were associated with increased incident IS risk in a dose–response pattern, except for TG (p for trend=0.18). Compared with respective reference groups, the upper tertile in cumulative TG burden increased 26% risk of IS (HR: 1.26; 95% CI: 1.02 to 1.55); the upper tertile in cumulative LDL-C burden increased 47% risk of IS (HR: 1.47; 95% CI: 1.25 to 1.73); the upper tertile in cumulative TC burden increased 33% risk of IS (HR: 1.33; 95% CI: 1.12 to 1.57); and the upper tertile in cumulative non-HDL-C burden increased 51% risk of IS (HR: 1.51; 95% CI: 1.28 to 1.80), after adjustment for confounding variables. However, this association was not observed in HDL-C and IS (HR: 1.09; 95% CI: 0.79 to 1.52) (table 2).
HRs and 95% CIs of IS according to different cumulative burden of lipid parameters
In addition, the adjusted HRs for the highest group versus lowest group of cumulative TG, TC, LDL and non-HDL burden were 1.61 (95% CI: 1.09 to 2.38), 2.01 (95% CI: 1.47 to 2.75), 1.88 (95% CI: 1.36 to 2.59) and 2.94 (95% CI: 2.06 to 4.19) for myocardial infarction. The relationship between HDL and myocardial infarction was not significant (online supplemental table S1). These results were similar with HRs for IS. After adjusted for confounding variables, only cumulative LDL was associated with all-cause death (HR: 1.14, 95% CI: 1.00 to 1.31) (online supplemental table S2).
Complementary and sensitivity analysis
Online supplemental table S3 showed the association between each cumulative burden of lipid profiles treated as continuous variables and IS. The adjusted HRs for the highest versus lowest quartiles of TG, TC, LDL and non-HDL were 1.43 (95% CI: 1.19 to 1.72), 1.41 (95% CI: 1.16 to 1.72), 1.46 (95% CI: 1.22 to 1.74) and 1.52 (95% CI: 1.26 to 1.83) for IS, respectively. However, this association was not significant in HDL-C and IS (HR: 0.94, 95% CI: 0.79 to 1.13). As shown above, results remained consistent when cumulative TG, LDL-C, TC, non-HDL-C and HDL-C burden were treated as continues variables.
(Online supplemental table S4 showed that cumulative LDL burden was still associated with IS incidence in participants with LDL <2.6 mmol/L in the last examination (HR: 1.12, 95% CI: 1.04 to 1.47).
Cumulative burden and residual risk of outcomes
Online supplemental table S5 presented baseline characteristics of participants with cumulative LDL-C burden=0 versus >0. Participants with cumulative LDL-C burden =0 and >0 were 16 600 and 27 236, respectively. Compared with those with cumulative LDL-C burden=0, participants with cumulative LDL-C >0 were elder, had higher levels of TG, TC, LDL-C, non-HDL-C, systolic blood pressure, higher proportion of smoker, history with diabetes mellitus and hypercholesterolaemia.
Among 16 600 participants with cumulative LDL-C burden=0, 315 (1.90%) cases of incident IS were identified. In participants with cumulative LDL-C burden=0, higher cumulative TG, TC and non-HDL-C burden were associated with increased residual risk for incident IS in a dose–response pattern (table 3). Compared with respective reference groups, the upper tertile in cumulative TG burden increased 65% risk of IS (HR: 1.65; 95% CI: 1.19 to 2.31); the upper tertile in cumulative TC burden increased 63% risk of IS (HR: 1.63; 95% CI: 1.03 to 2.57) and the upper tertile in cumulative non-HDL-C burden increased 57% risk of IS (HR: 1.57; 95% CI: 1.06 to 2.32), after adjustment for confounding variables. Cumulative HDL burden was still not significantly associated with IS (HR: 0.98; 95% CI: 0.56 to 1.72).
Lipids burden related residual risk beyond LDL-C in participants with low cumulative LDL-C burden
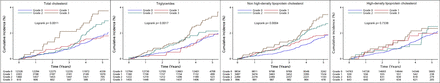
Kaplan-Meier cumulative event curves likewise suggested a higher risk for incident IS in higher tertiles of cumulative TG, TC and non-HDL-C burden especially at late time points, except for cumulative HDL-C burden (figure 2).
Kaplan-Meier curves of incidence of ischaemic stroke by cumulative burden of lipid parameters in participants with low cumulative low-density lipoprotein cholesterol burden.
Discussion
In this large community-based prospective study of 47 832 Chinese adults, we observed that: (1) participants with the higher cumulative burden of TG, TC, LDL-C and non-HDL-C burden, as compared with those in low group, was more likely to develop stroke. (2) While this relationship did not exist in cumulative burden of HDL-C, higher cumulative burden of TG, TC, LDL-C and non-HDL-C burden, except for cumulative HDL-C burden, was consistently associated with higher subsequent IS risk in participants with low cumulative burden of LDL-C. Primary prevention for IS should not only focus on the magnitude of lipid burden at one time point but also take the prior duration of cumulative lipid profiles exposure into consideration.
Because the natural history of atherosclerosis is prolonged, the risk of clinical events rises exponentially late in life.26 Incorporating both the LDL-C concentration and exposure duration into a single risk parameter for future cardiovascular events is intuitively appealing. A previous study derived from Framingham Offspring Cohort data suggested that cumulative exposure to hyperlipidaemia in young adulthood increases the subsequent risk of coronary heart disease in a dose-dependent fashion.26
Cumulative exposure to hyperlipidaemia in young adulthood increases the subsequent risk of coronary heart disease in a dose-dependent fashion. In patients with systemic lupus erythematosus, first-available TC was not predictive of cardiovascular disease among patients, in whom measures reflecting cumulative exposure over time are better able to quantify cardiovascular disease risk.27 These studies stress the importance of cumulative exposure on lipid levels.
Our study extended the previous works by not only showing the cumulative burden of LDL-C, but also other ApoB-containing lipoproteins.28 However, Mendelian randomisation studies have not indicated any causality between HDL-C and cardiovascular disease.29–31 Cumulative burden of HDL-C also showed no significant relationship with incident IS in the current study.
Moreover, clinical trials evaluating lipid-lowering therapy for primary prevention have mostly been limited to intermediate-risk and high-risk groups32–34; studies evaluating the association of other lipid profiles with incident IS, specifically in the group of low LDL-C burden, are limited despite the fact that this group accounts for a rather high percentage of the population. Clinical evidence suggests that the residual cardiovascular risk observed in patients with well-controlled LDL-C levels can be, in part, explained by residual lipid risk factor.11 35 Residual hypertriglyceridaemia occurs over one-fifth (5.5 million) of US adults with diabetes, including those on statin therapy and well-controlled LDL-C. Over three quarters of adults with diabetes with hypertriglyceridaemia are at moderate or high 10-year risk for atherosclerotic cardiovascular disease.36 Using the database of our Chronic Heart Failure Analysis and Registry in the Tohoku District 2 study, the largest scale cohort study of cardiovascular patients in Japan, a previous study indicates that higher triglyceride levels were associated with higher incidence of recurrent myocardial infarction in patients with LDL <100 mg/dL.37 Our study confirmed the association between cumulative burden of other lipid profiles and IS in individuals with low risk evaluated by LDL-C.
Continued exposure to ApoB-containing lipoproteins leads to additional particles being retained over time in the artery wall, and to the growth and progression of atherosclerotic plaques. Once the size of the total plaque burden exceeds this threshold, a person is at risk of experiencing an acute vascular event.12 Because the risk of cardiovascular events depends on the cumulative lifetime exposure to LDL-C and other ApoB-containing lipoproteins, primary prevention strategies designed to lower lipids closer to optimal levels should be initiated in early adulthood to minimise the cumulative lifetime exposure to atherogenic lipoproteins.
The strengths of this study include its prospective design, the large population with a complete follow-up of stroke and repeated assessment of various lipids measurements. However, our study has several limitations. First of all, in this subanalysis of the Kailuan study, the information on four surveys (2006–2007, 2008–2009, 2010–2011 and 2012–2013) was used to calculate the cumulative burden of lipid profiles. The 6 year prior duration of exposure to cumulative lipid profiles recorded in this study, to a certain extent, may be not quite substantial to represent the effect of exposure duration. Second, the associations of lipids profiles and stroke vary by stroke subtypes according to previous studies2; however, we cannot further explore the potential effect of lipids variability on different subtypes of IS. Finally, the Kailuan Study was not designed to be nationally representative. The study was conducted in Tangshan city, a city of northern China. Therefore, it may limit the generalisability of the conclusions to other settings and populations.
Conclusions
In conclusion, the present study suggested that cumulative burden of lipid profiles were associated with incident IS, except for HDL-C. Cumulative burden of TG, TC, LDL-C and non-HDL-C burden was consistently associated with higher subsequent IS risk even in participants with low cumulative burden of LDL-C. These findings may have implications for future cholesterol treatment paradigms.
Data availability statement
Data are available upon reasonable request. Data are available to researchers on request for purpose of reproducing the results or replicating the procedure by directly contacting the corresponding author.
Ethics statements
Patient consent for publication
Ethics approval
The study was approved by the central institutional review board at Beijing Tiantan Hospital. All patients or the designated relatives gave written consent when enrolled.
References
Supplementary material
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors Study concept and design: LD, JX, SW and YW. Acquisition, analysis or interpretation of data: YZ, AW, ZC and JM. Drafting of the manuscript: LD. Critical revision of the manuscript for important intellectual content: LD, JX, HL, XM, SW and YW. Statistical analysis: YZ and AW. Study supervision: SW and YW. All authors approved the final version of the manuscript.
Funding This work was supported by a grant from National Key R&D programme of China (2017YFC1310902).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.