Article Text
Abstract
There are several previous studies on the association of vitamin E with prevention of stroke but the findings remain controversial. We have conducted a systematic review, meta-analysis together with trial sequential analysis of randomised controlled trials to evaluate the effect of vitamin E supplementation versus placebo/no vitamin E on the risk reduction of total, fatal, non-fatal, haemorrhagic and ischaemic stroke. Relevant studies were identified by searching online databases through Medline, PubMed and Cochrane Central Register of Controlled Trials. A total of 18 studies with 148 016 participants were included in the analysis. There was no significant difference in the prevention of total stroke (RR (relative risk)=0.98, 95% CI 0.92–1.04, p=0.57), fatal stroke (RR=0.96, 95% CI 0.77–1.20, p=0.73) and non-fatal stroke (RR=0.96, 95% CI 0.88–1.05, p=0.35). Subgroup analyses were performed under each category (total stroke, fatal stroke and non-fatal stroke) and included the following subgroups (types of prevention, source and dosage of vitamin E and vitamin E alone vs control). The findings in all subgroup analyses were statistically insignificant. In stroke subtypes analysis, vitamin E showed significant risk reduction in ischaemic stroke (RR=0.92, 95% CI 0.85–0.99, p=0.04) but not in haemorrhagic stroke (RR=1.17, 95% CI 0.98–1.39, p=0.08). However, the trial sequential analysis demonstrated that more studies were needed to control random errors. Limitations of this study include the following: trials design may not have provided sufficient power to detect a change in stroke outcomes, participants may have had different lifestyles or health issues, there were a limited number of studies available for subgroup analysis, studies were mostly done in developed countries, and the total sample size for all included studies was insufficient to obtain a meaningful result from meta-analysis. In conclusion, there is still a lack of statistically significant evidence of the effects of vitamin E on the risk reduction of stroke. Nevertheless, vitamin E may offer some benefits in the prevention of ischaemic stroke and additional well-designed randomised controlled trials are needed to arrive at a definitive finding. PROSPERO registration number: CRD42020167827.
- stroke
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Stroke is defined as rapidly developing focal (or global) disturbance of cerebral function, including cerebral infarction, intracerebral haemorrhage and subarachnoid haemorrhage. It usually occurs with one or more clinical signs, lasting for more than 24 hours or leading to death, with no apparent cause other than it being of vascular origin.1 Globally, 84.4% of all strokes are ischaemic and 15.6% are haemorrhagic. According to The Global Burden of Diseases, Injuries, and Risk Factors Study, there are 5.5 million stroke deaths annually around the world and it is the second leading cause of death globally.2 Stroke-related morbidity remains high. It is estimated that the total annual cost due to stroke will increase to $240.67 billion by 2030, including both stroke-related medical costs and indirect annual costs attributable to loss of productivity.3 Thus, proper stroke management and preventive measures are crucial in helping to reduce escalating healthcare costs. Notwithstanding that both modifiable and non-modifiable risk factors have been widely associated with stroke, there is evidence that certain types of diet and nutrition are linked with the incidence or prevention of stroke.4 5
Vitamin E is a lipid-soluble antioxidant that diminishes the rate of oxidative stress, an important component in the pathogenesis of atherosclerosis. Oxidised phospholipids and other lipid oxidation products (lipid peroxidation) permit build-up of plaque in arteries and cause atherogenesis.6 By scavenging the reactive oxygen species, modifying vascular endothelium vasodilator responsiveness7 and reducing platelet aggregation,8 as well as preserving cell membranes, vitamin E plays a crucial role in inhibiting formation of atherosclerosis.9 Therefore, for populations in which atherosclerosis in major intracranial arteries accounts for a high prevalence of stroke, such as African-American, Asian (Chinese, Japanese, South Korean, Indian) and Hispanic,10 vitamin E supplementation may aid in stroke prevention.
Previous prospective cohort studies have indicated association between a high intake of vitamin E and the prevention of cardiovascular disease (CVD).11–13 A community study (Aric Study) also hypothesised that vitamin E may protect against atherosclerosis and thus prevent stroke.14 From a subgroup analysis of a cancer prevention study, patients with hypertension showed a reduction in the risk of ischaemic stroke with vitamin E supplementation. Additionally, similar results were also found in hypertensive patients with diabetes.15
To date, there have been several systematic reviews and meta-analyses of studies investigating the association of vitamin E with stroke prevention.16–20 However, the results are inconsistent. The discrepancy in the outcomes is mainly due to the pathological subtypes of stroke, namely ischaemic stroke and haemorrhagic stroke. In addition, we added quality assessment on all of the included studies since the previous systematic reviews had either no quality assessment or incomplete quality assessment. Furthermore, we also included trial sequential analysis (TSA) because previously there had not been any quantitative attempt to summarise the precise effect of vitamin E on prevention of stroke. Hence, the objective of this systematic review and meta-analysis with TSA was to assess the effect of vitamin E on stroke prevention based on a sufficient sample size and with adequate reliability of the conclusions.
Methods
This study was registered with PROSPERO and the local National Medical Research Register (NMRR-20-1197-55340) and performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.21
Literature search
Two investigators (HCL and KWL) independently searched through three major citation databases, namely Medline, PubMed, as well as Cochrane Central Register of Controlled Trials, and included all relevant articles published from inception to 12 February 2020. We limited our search to English language articles and studies relating only to humans. We also used reverse-forward citation (hand searches) from the included studies to search for relevant studies. We used a combination of search terms as follows (antioxidant OR anti-oxidant OR vitamin* OR vitamin E OR tocopherol OR tocotrienol) AND (stroke OR cerebral vascular OR cerebrovascular OR transient ischemic attack OR brain attack OR brain isch*emia OR intracranial bleed* OR intracranial h*emorrhage OR brain h*emorrhage OR cardiovascular) AND (control* trial* OR clinical trial* OR random*). Study authors were contacted for clarification where necessary.
Intervention and control group definitions
The intervention group was defined as subjects who were taking vitamin E supplementation while the control group was defined as subjects who were taking a placebo or receiving no treatment at all.
Study selection
All articles obtained via the database searches were imported into Endnote programme X9 version, merged and any duplicate publications were removed. Titles and abstracts were screened independently by three investigators (HCL, CYO, DRC). Full-text articles were retrieved and independently reviewed by the same investigators to assess eligibility. We also manually performed reverse-forward citation of the identified studies. Any disagreements were resolved by consensus. We included randomised controlled trials (RCT) comparing the effect of vitamin E supplementation on the incidences of stroke and its subtypes. Studies were excluded if there was no access to full text, and/or there were multiple papers reporting on the same trial (we chose either the original paper or the one with the most relevant information or outcomes).
Data extraction
Two investigators (HCL and RL) independently reviewed and extracted relevant data from each of the included articles using a standard data extraction template. Extracted data included the following variables: study characteristics (first author, trial’s name, publication year, country and study design), baseline characteristics of the study population, type of prevention, trial duration, intervention and trial outcome measures (number of total stroke, fatal stroke, non-fatal stroke, ischaemic stroke and haemorrhagic stroke). All discrepancies and disagreements were resolved through consensus.
Data syntheses
The results were pooled if comparable outcome data were available from two or more studies. Meta-analysis was performed with Review Manager software22 using a random-effects model to produce the study risk ratio and their respective 95% CIs with a two‐sided p value of <0.05 considered as statistically significant. Statistical heterogeneity between studies was assessed using the I2 index (low was <25%, moderate 25%–50% and high >50%), and the sources of heterogeneity were explored if present.23 In addition, a leave-one-out meta-analysis was performed as a sensitivity analysis to determine how individual studies influenced the overall estimate of the rest of the studies with exactly one study being left out each time.24 We also performed TSA for the outcome with the random‐effects (DerSimonian and Laird) model using the TSA software package.25 We set α level of 0.05 (two-sided) and a β level of 0.20 (80% power), and the control event proportions were calculated from the control arm of the trials. TSA contributed to a better control of type I26 27 and type II errors27 and provided clarification if more trials were needed.
Risk of bias assessment
Quality of the included trials was independently assessed by two investigators (HCL and RL) for risk of bias using the Cochrane Risk of Bias Tool.28 29 Assessment was done across five domains of bias including the bias arising from the randomisation process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome and bias in selection of the reported results. Questions within each domain were answered as ‘Yes’ or ‘Probably Yes’ or ‘No’ or ‘Probably No’ or ‘No information’. The responses were used to indicate whether there is low risk (proper methods taken to reduce bias), some concern (insufficient information provided to determine the bias level), or high risk (improper methods creating bias). All discrepancies and disagreements were resolved through consensus. Risk of bias assessment across studies was reported too with publication bias. The bias was assessed by using the funnel plot and was quantified by using Egger and Begg’s tests.30 31
Grading of the evidence
The quality of evidence for each trial outcome was subsequently assessed using the Grading of Recommendation, Assessment, Development and Evaluation (GRADE) framework methodology.32–36 The certainty of evidence was assessed as high, moderate, low or very low based on the basic design of the studies, the limitations in design or execution due to the risk of bias in the studies, the indirectness of the evidence, the inconsistency of results across studies, the imprecision of results and other considerations.
Results
Search results
Figure 1 shows the literature search and selection process. We identified a total of 3609 studies after removing duplicates, 3543 of which were excluded based on review of the title and/or abstract. The remaining 66 studies were retrieved and reviewed in full and 48 were excluded based on selection criteria. A total of 18 studies met the eligibility criteria and were included in the final analyses.37–54
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the literature screening process. RCT, randomised controlled trials.
Trials characteristics
The characteristics of 18 included randomised controlled trials are summarised in table 1. The sample sizes of these trials ranged from 100 to 39 876 subjects, and the total number of subjects in the trials was 148 016 with 74 000 randomised to vitamin E and 74 016 to control arm. Out of seven continents, seven studies were conducted in Europe,37–40 43 47 48 five in North America,46 51–54 three in Asia44 45 49 and three were international and not continent-specific.41 42 50 In two trials, vitamin E was compared with no vitamin E40 48 while the remaining trials compared vitamin E to a placebo. All the studies were comprised mixed genders except for four studies which respectively involved only female52 53 and male37 46 subjects. Subjects from all studies were with comorbidity except three studies37 51 53 in which the subjects were healthy individuals or without known serious illness. Across the studies, α-tocopherol was used. Eight studies used vitamin E from a natural source38 41 42 44 49 50 52 53 while another eight studies used vitamin E from a synthetic source.37 39 40 43 45 46 48 51 All the studies had a trial duration that ranged from 10 days to 10.9 years.
Characteristics of trials
Sensitivity and publication bias analysis
We performed sensitivity analyses of vitamin E vs placebo/no vitamin E for total stroke, fatal stroke, non-fatal stroke, haemorrhagic stroke and ischaemic stroke as outcome measures to reveal the studies which affected the pooled relative risk. We only excluded a study from the meta-analyses if the study was high in heterogeneity and with publication bias.
For haemorrhagic stroke, there were two RCTs43 53 while for ischaemic stroke, three RCTs37 49 52 were found to influence the summary estimate respectively. Nevertheless, since there was no significant publication bias based on the funnel plot, Egger’s test and Begg’s test (haemorrhagic stroke: Egger’s test, p=0.251; Begg’s test p=0.293, and ischaemic stroke: Egger’s test, p=0.228; Begg’s test p=0.176) and the heterogeneity were low for both outcomes (haemorrhagic stroke: I2=0%, and ischaemic stroke: I2=5%), we did not exclude any studies from the meta-analyses.
Effect of vitamin E on stroke and its subgroup analysis
The effect of vitamin E on total stroke and its subgroup analysis by type of prevention (primary or secondary), source of vitamin E (synthetic or natural), dosage of vitamin E (high if 300 IU or more; or low if less than 300 IU) and vitamin E alone (without other pharmacological and/or non-pharmacological intervention) are shown in table 2. There is no significant reduction in the risk of total stroke observed in those taking vitamin E supplement as compared with control arm (RR=0.98, 95% CI 0.92–1.04, p=0.57) among all subjects. Similar results were also seen when we compared the effect of vitamin E on primary with secondary prevention of stroke, risk reduction of stroke between synthetic and natural vitamin E, high versus low dosage of vitamin E and when vitamin E alone was used.
Relative risks of the effects of vitamin E on stroke for the pooled population and its subgroup analysis
Supplemental material
Supplementation with vitamin E does not significantly reduce the risk of fatal stroke (RR=0.96, 95% CI 0.77–1.20, p=0.73) or non-fatal stroke (RR=0.96, 95% CI 0.88–1.05, p=0.35). In their respective subgroup analysis, we observed that all the findings were insignificant as well.
In stroke subtypes analysis, vitamin E showed significant risk reduction in ischaemic stroke (RR=0.92, 95% CI 0.85–0.99, p=0.04) but not in haemorrhagic stroke (RR=1.17, 95% CI 0.98–1.39, p=0.08).
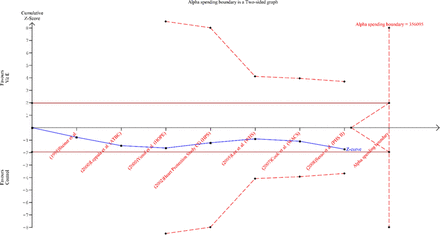
For the risk reduction of vitamin E on total stroke and fatal stroke, the boundary TSA was ignored because too little information was used, while 3 542 181 and 8 193 891 subjects, respectively are needed for stable conclusions for total stroke and fatal stroke prevention (figures 2 and 3). For the risk reduction of vitamin E on non-fatal stroke and haemorrhagic stroke, the cumulative Z curve (blue curve) did not reach either the conventional boundary or the α-spending boundary. This indicates that there was no significant difference between the subjects treated with vitamin E and without vitamin E, and the required information size of 1 159 873 and 356 095 subjects, respectively are needed (figures 4 and 5). Since the cumulative Z curves did not cross the trial sequential boundaries for benefit, harm or futility, we cannot exclude the risks of random errors based on traditional naïve meta-analysis.
Trial sequential analysis on the effect of vitamin E compared with control on total stroke prevention.
Trial sequential analysis on the effect of vitamin E compared with control on fatal stroke prevention.
Trial sequential analysis on the effect of vitamin E compared with control on non-fatal stroke prevention.
Trial sequential analysis on the effect of vitamin E compared with control on haemorrhagic stroke prevention.
Conversely, for the risk reduction of vitamin E on ischaemic stroke, the cumulative Z curve initially crossed the conventional boundary for benefit after the first trial, then regressed, and bounced back at the end after other trials were added. Nevertheless, the cumulative Z curve did not cross the trial sequential monitoring boundary, and 212 626 patients are needed to reduce spurious conclusions (figure 6). Therefore, the preventive effect of vitamin E on ischaemic stroke remains inconclusive based on the current sample size available.
Trial sequential analysis on the effect of vitamin E compared with control on ischaemic stroke prevention.
Risk of bias within studies
We evaluated the risk of bias for each included study. All the studies were randomised but we had some concerns in our evaluation when there was no information present addressing such issues as how the randomisation of sequence had been conducted, how allocation sequences had been concealed and so on.41 45 54 We considered that there may be some concern for bias to deviation from intended intervention arising from the studies when subjects were aware of their assigned intervention during the trial.40 48 With respect to the attrition bias, most of the studies provided inadequate information as there were large numbers of subjects who were lost during follow-up, which led to the bias of missing outcome data.39 41 42 44–52 54 Regarding the bias in measurement of the outcome, all the studies were at low risk of bias because the methods of measuring the outcome were appropriate, and ascertainment of the outcome was the same between the intervention and control groups. For the risk of bias from selection of the reported result, the majority of the studies were low in bias, except one study37 which may not have performed according to the pre-specified analysis plan. In that study,37 some subjects were excluded after randomisation. In another study,54 the statistical method and analysis plan were not mentioned. All in all, three studies38 43 53 were considered to have an overall low risk of bias, fourteen studies37 39–42 44–52 had some concern of risk of bias overall and one study54 was considered to have an overall high risk of bias. A graph and a summary of risk of bias are shown in online supplemental figures S16 and S17.
GRADE assessment
By using GRADE, we rated the quality of evidence as high for vitamin E effect on fatal stroke and non-fatal stroke; moderate for total stroke and ischaemic stroke owing to a downgrade for risk of bias as most of the included studies were ruled out for low risk of bias; and very low for haemorrhagic stroke due to very serious imprecision because the number of events was small and the confidence intervals were wide. GRADE evidence profiles and a summary of findings are shown in online supplemental table S1.
Discussion
This study revealed that vitamin E supplementation significantly reduced the risk of ischaemic stroke by 8% and there was no significant heterogeneity among the trials (p value for heterogeneity=0.39). Although the exact mechanism of action for this finding is unknown, we conjecture that it could be attributed to its antioxidative activity,55 antiplatelet action8 and antiatherogenic properties.6 This can be seen in studies that show that a diet predominantly composed of fruits and vegetables, high in antioxidant vitamins, is associated with lower incidence of stroke.56 In addition, a randomised controlled trial, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study15 37 indicated a reduction in ischaemic stroke of about 14% (95% CI 0.75–0.99, p=0.03) with vitamin E supplementation. Nevertheless, we performed TSA which demonstrated that the available data were insufficient to draw firm conclusions regarding the superiority of vitamin E in preventing the risk of ischaemic stroke. Hence, we believe additional well-designed RCTs are necessary to provide a more accurate and reliable outcome.
Our meta-analysis did not detect any significant difference from vitamin E supplementation in the risk reduction of haemorrhagic stroke. The finding was significantly different from Schürks et al which showed an increased risk of 22% (95% CI 1.00–1.48, p=0.045).16 This may be due to the incorporation of an extra two RCTs43 54 in our study. Since the heterogeneity is low (I2=0%), we decided to incorporate the trials in our pooled risk reduction result. On top of that, TSA indicated that previous meta-analysis16 may have led to random errors and the current evidence for a vitamin E preventive effect on haemorrhagic stroke was insufficient to draw meaningful conclusions. Therefore, even though vitamin E is known for its antiplatelet57 and anticoagulant effect58 due to its interference with activation of the vitamin K dependent clotting factor, the conclusion that vitamin E increases the risk of haemorrhagic stroke cannot be established.
Furthermore, our meta-analyses showed no significant preventive action of vitamin E in total stroke, fatal stroke and non-fatal stroke. Our findings confirm a result published in the journal of the American Heart Association59 which reported that there was no statistical difference favouring the vitamin E group compared with the control group in stroke prevention. However, TSA indicated that the current evidence for total stroke, fatal stroke and non-fatal stroke was insufficient to draw solid conclusions.
Based on the dietary reference intakes framework developed by US and Canadian scientists, 1 IU of the natural form is equivalent to 0.67 mg of α-tocopherol and 0.45 mg of α-tocopherol in synthetic form.60 Previous studies also showed that a relatively high dose of α-tocopherol of more than 300 IU daily aids in the prevention of CVD.61 62 Thus, we further investigated the effect of vitamin E on the prevention of stroke by conducting subgroup analysis to look at the outcome by types of prevention, as well as source and dosage of vitamin E. The results showed no significant difference regardless of whether it is for primary or secondary prevention, from a synthetic or natural source, or whether high or low doses of vitamin E supplementation were used.
We added another analysis to look at the preventive effect of vitamin E on stroke when it is used alone without other pharmacological and/or non-pharmacological intervention. This helps to exclude factors contributed by the synergistic or mixed effect of other interventions with vitamin E. The outcome was not significant. We included 4 RCTs that meet the criteria and the results we obtained only reflect on total stroke. Thus, more RCTs with other outcome measures of stroke are needed for a stronger conclusion.
Limitations and future research recommendation
There are several limitations in the current study. First, for some of the studies, stroke or CVD were not primary outcomes. Therefore, the trials design may not provide sufficient power to detect a change in these outcomes. Second, the participants have different risk factors to stroke, such as differences in smoking status, body mass index, alcohol consumption, lifestyle, diet and so forth. All of these are associated with a potential to increase or reduce the risk of stroke. Third, the results of subgroup analyses may not be robust enough due to the limited number of studies included. Fourth, the included studies were mainly conducted in developed countries such as the USA and European countries. Other than differences like race, lifestyle, dietary habits and so on, populations of developed countries are usually without known vitamin deficiency and have lower incidence of stroke. Hence, it is difficult to generalise the results to populations in other parts of the world. Fifth, ischaemic stroke subgroup analysis was not carried out as the papers included were too few in number to provide sufficient statistical power to achieve a worthwhile result.63 Also, due to the lack of information to standardise available data, this study did not perform meta-analysis for the duration of vitamin E supplementation with respect to its preventive effect of stroke. Finally, we lacked sufficient sample size from RCTs to conclusively support our hypotheses and any conclusions we derived are made with caution. Future research with larger stroke-oriented randomised clinical trials and with more homogenised characteristics of participants from different parts of the world are needed to prove the efficacy of vitamin E supplementation in stroke prevention.
Conclusion
In conclusion, the findings of this review demonstrate that vitamin E cannot be unequivocally shown to prevent stroke. However, it may be beneficial to prevent ischaemic stroke and additional well-designed RCTs are needed to draw a conclusive statement. Vitamin E is regularly consumed as a supplement without evidence of a statistically significant increase in the incidence of adverse events. It only serves as an adjunct to, rather than as a replacement for medication due to the greater risk reduction of stroke achieved by medication. Therefore, the use of vitamin E supplementation remains debatable until future trials provide more reliable evidence.
Acknowledgments
We would like to thank the Director General of Health Malaysia for his permission to publish this article. We also would like to thank Prof. Dr. Liew Su-May (Department of Primary Care Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia) for her guidance.
References
Supplementary material
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors Conceptualisation: HCL, KWL and NAKK; methodology: HCL, RL, KWL, CYO, DRC and NAKK; software: HCL, RL, KWL, CYO and DRC; validation: IL, KHY and NAKK; formal analysis: HCL, RL, KWL; investigation: IL, KHY and NAKK; resources: IL, KHY and NAKK; data curation: IL, KHY and NAKK; writing-original draft preparation: HCL, RL; writing-review and editing: IL, KHY and NAKK; visualisation: HCL, KWL; supervision: KHY; project administration: IL; funding acquisition: not applicable. All authors have read and agreed to the published version of the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.